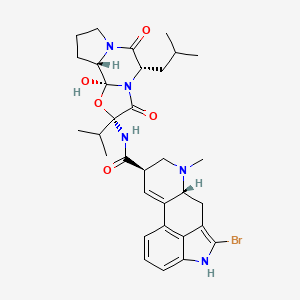
Bromocriptin
Übersicht
Beschreibung
Bromocriptine is an ergoline derivative and a dopamine agonist. It was originally marketed under the brand name Parlodel and has since been sold under various other names. Bromocriptine is primarily used in the treatment of pituitary tumors, Parkinson’s disease, hyperprolactinemia, neuroleptic malignant syndrome, and type 2 diabetes . It was patented in 1968 and approved for medical use in 1975 .
Wissenschaftliche Forschungsanwendungen
Bromocriptin hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Biologie: this compound wird verwendet, um die Funktionen von Dopaminrezeptoren und Signalwege zu untersuchen.
Medizin: Es wird in großem Umfang zur Behandlung von Erkrankungen wie Hyperprolaktinämie, Parkinson-Krankheit und Typ-2-Diabetes eingesetzt
5. Wirkmechanismus
This compound übt seine Wirkungen aus, indem es als Dopamin-D2-Rezeptor-Agonist wirkt. Es bindet an Dopaminrezeptoren im Gehirn und hemmt die Freisetzung von Prolaktin aus der Hypophyse. Dieser Mechanismus ist vorteilhaft bei der Behandlung von Erkrankungen wie Hyperprolaktinämie und Parkinson-Krankheit. Im Falle von Typ-2-Diabetes trägt this compound zur Verbesserung der Blutzuckerkontrolle bei, indem es den Dopaminspiegel im Hypothalamus erhöht, was wiederum die Insulinresistenz und die Glukoseproduktion in der Leber reduziert .
Ähnliche Verbindungen:
Cabergolin: Ein weiterer Dopaminagonist, der zur Behandlung von Hyperprolaktinämie und Parkinson-Krankheit eingesetzt wird.
Pergolid: Wird zur Behandlung der Parkinson-Krankheit eingesetzt.
Quinagolid: Ein nicht-ergoliner Dopaminagonist, der zur Behandlung von Hyperprolaktinämie eingesetzt wird.
Vergleich: this compound ist einzigartig in seiner schnell freisetzenden Formulierung, die ein schnelles Einsetzen der Wirkung ermöglicht. Es wird auch als Begleittherapie bei Typ-2-Diabetes eingesetzt, ein Merkmal, das bei anderen Dopaminagonisten nicht üblich ist. Cabergolin hingegen hat eine längere Halbwertszeit und wird aufgrund seiner weniger häufigen Dosierung oft bevorzugt .
Wirkmechanismus
Target of Action
Bromocriptine is a dopamine D2 receptor agonist . It primarily targets the dopamine receptors in the brain, particularly in the basal ganglia , hypothalamus , and midbrain . These receptors play a crucial role in regulating various physiological functions, including prolactin secretion, motor control, and certain aspects of behavior .
Mode of Action
Bromocriptine interacts with its targets by activating postsynaptic dopamine receptors in the tuberoinfundibular and nigrostriatal pathways . In the tuberoinfundibular pathway, this activation leads to the inhibition of pituitary prolactin secretion , which can be beneficial in treating conditions associated with hyperprolactinemia . In the nigrostriatal pathway, bromocriptine enhances coordinated motor control , which is particularly useful in managing Parkinsonian Syndrome .
Biochemical Pathways
Bromocriptine’s action on the dopamine receptors affects several biochemical pathways. It is believed to augment low hypothalamic dopamine levels and inhibit excessive sympathetic tone within the central nervous system (CNS), resulting in a reduction in postmeal plasma glucose levels due to enhanced suppression of hepatic glucose production . Bromocriptine also reduces fasting and postmeal plasma free fatty acid (FFA) and triglyceride levels .
Result of Action
The molecular and cellular effects of bromocriptine’s action are diverse. It has been shown to reduce the expression of Ki67 protein in the endometrium of women with adenomyosis, indicating a potential anti-proliferative effect . Bromocriptine also significantly inhibits the proliferative and migrative abilities of endometrial stromal cells derived from women with adenomyosis . In the context of type 2 diabetes, bromocriptine reduces the mRNA expression of the damage-associated molecular pattern (DAMP)-activated pro-inflammatory pathway initiators TLR2 and TLR4 .
Action Environment
Environmental factors can influence the action, efficacy, and stability of bromocriptine. For instance, long-term treatment with bromocriptine has minimal or no harmful effects on renal, hepatic, cardiac, or hematologic functions . Moreover, bromocriptine treatment has been shown to reduce blood glucose and body mass index (BMI), which are major cardiovascular risk factors in type 2 diabetes mellitus patients . .
Biochemische Analyse
Biochemical Properties
Bromocriptine is a partial agonist of the dopamine D2 receptor . It also interacts with other dopamine receptors and with various serotonin and adrenergic receptors . Bromocriptine has additionally been found to inhibit the release of glutamate by reversing the GLT1 glutamate transporter .
Cellular Effects
Bromocriptine blocks the release of a hormone called prolactin from the pituitary gland . Prolactin affects the menstrual cycle and milk production . Bromocriptine is used to treat certain menstrual problems in women and stops milk production in some men and women who have abnormal milk leakage .
Molecular Mechanism
Bromocriptine stimulates centrally-located dopaminergic receptors resulting in a number of pharmacologic effects . Five dopamine receptor types from two dopaminergic subfamilies have been identified . The dopaminergic D1 receptor subfamily consists of D1 and D5 subreceptors, which are associated with dyskinesias .
Temporal Effects in Laboratory Settings
In laboratory settings, bromocriptine use was associated with a modest but statistically significant decline in temperature, with nadir at 72 h post initiation . This suggests that the effects of bromocriptine can change over time in a laboratory setting.
Dosage Effects in Animal Models
In animal models, studies conducted on bromocriptine have shown that stimulating D2 receptors may enhance working memory in rodents, whereas inhibiting these receptors could have the opposite effect, reducing working memory performance .
Metabolic Pathways
Bromocriptine is completely metabolized by the liver, primarily by hydrolysis of the amide bond to produce lysergic acid and a peptide fragment, both inactive and non-toxic . Bromocriptine is metabolized by cytochrome P450 3A4 and excreted primarily in the feces via biliary secretion .
Transport and Distribution
Bromocriptine is rapidly absorbed (about 28% to 37% of the total dose) after oral administration . It is metabolized by cytochrome P450 3A4 and excreted primarily in the feces via biliary secretion .
Vorbereitungsmethoden
Synthetische Wege und Reaktionsbedingungen: Bromocriptin wird durch eine Reihe chemischer Reaktionen synthetisiert, ausgehend von Mutterkornalkaloiden. Die Herstellung von this compound-Mesylat beinhaltet das Lösen von this compound in Methanol, gefolgt von der Zugabe einer wässrigen Lösung von Methansulfonsäure. Die Mischung wird gerührt, gekühlt, filtriert und mit Ethanol umkristallisiert, um this compound-Mesylat zu erhalten .
Industrielle Produktionsmethoden: Die industrielle Produktion von this compound folgt ähnlichen Schritten, jedoch in größerem Maßstab. Der Prozess beinhaltet eine präzise Kontrolle der Reaktionsbedingungen, wie z. B. Temperatur und pH-Wert, um eine hohe Ausbeute und Reinheit des Endprodukts zu gewährleisten .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Bromocriptin unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann unter bestimmten Bedingungen oxidiert werden, um verschiedene Metaboliten zu bilden.
Reduktion: Reduktionsreaktionen können das Bromatom im Molekül modifizieren.
Substitution: this compound kann Substitutionsreaktionen eingehen, bei denen das Bromatom durch andere funktionelle Gruppen ersetzt wird.
Häufige Reagenzien und Bedingungen:
Oxidation: Häufige Oxidationsmittel sind Kaliumpermanganat und Wasserstoffperoxid.
Reduktion: Reduktionsmittel wie Lithiumaluminiumhydrid können verwendet werden.
Substitution: Halogenaustauschreaktionen können mit Reagenzien wie Natriumiodid durchgeführt werden.
Hauptprodukte: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, hängen von den jeweiligen Bedingungen und Reagenzien ab. So kann die Oxidation zur Bildung verschiedener hydroxylierter Metaboliten führen .
Vergleich Mit ähnlichen Verbindungen
Cabergoline: Another dopamine agonist used to treat hyperprolactinemia and Parkinson’s disease.
Pergolide: Used in the treatment of Parkinson’s disease.
Quinagolide: A non-ergoline dopamine agonist used to treat hyperprolactinemia.
Comparison: Bromocriptine is unique in its quick-release formulation, which allows for rapid onset of action. It is also used as an adjunct therapy for type 2 diabetes, a feature not commonly seen with other dopamine agonists. Cabergoline, on the other hand, has a longer half-life and is often preferred for its less frequent dosing schedule .
Eigenschaften
IUPAC Name |
(6aR,9R)-5-bromo-N-[(1S,2S,4R,7S)-2-hydroxy-7-(2-methylpropyl)-5,8-dioxo-4-propan-2-yl-3-oxa-6,9-diazatricyclo[7.3.0.02,6]dodecan-4-yl]-7-methyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H40BrN5O5/c1-16(2)12-24-29(40)37-11-7-10-25(37)32(42)38(24)30(41)31(43-32,17(3)4)35-28(39)18-13-20-19-8-6-9-22-26(19)21(27(33)34-22)14-23(20)36(5)15-18/h6,8-9,13,16-18,23-25,34,42H,7,10-12,14-15H2,1-5H3,(H,35,39)/t18-,23-,24+,25+,31-,32+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OZVBMTJYIDMWIL-AYFBDAFISA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)CC1C(=O)N2CCCC2C3(N1C(=O)C(O3)(C(C)C)NC(=O)C4CN(C5CC6=C(NC7=CC=CC(=C67)C5=C4)Br)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)C[C@H]1C(=O)N2CCC[C@H]2[C@]3(N1C(=O)[C@](O3)(C(C)C)NC(=O)[C@H]4CN([C@@H]5CC6=C(NC7=CC=CC(=C67)C5=C4)Br)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H40BrN5O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
22260-51-1 (mesylate (salt)) | |
| Record name | Bromocriptine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0025614033 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID1022687 | |
| Record name | Bromocriptine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
654.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Bromocriptine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015331 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
8.58e-02 g/L | |
| Record name | Bromocriptine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015331 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The dopamine D2 receptor is a 7-transmembrane G-protein coupled receptor associated with Gi proteins. In lactotrophs, stimulation of dopamine D2 receptor causes inhibition of adenylyl cyclase, which decreases intracellular cAMP concentrations and blocks IP3-dependent release of Ca2+ from intracellular stores. Decreases in intracellular calcium levels may also be brought about via inhibition of calcium influx through voltage-gated calcium channels, rather than via inhibition of adenylyl cyclase. Additionally, receptor activation blocks phosphorylation of p42/p44 MAPK and decreases MAPK/ERK kinase phosphorylation. Inhibition of MAPK appears to be mediated by c-Raf and B-Raf-dependent inhibition of MAPK/ERK kinase. Dopamine-stimulated growth hormone release from the pituitary gland is mediated by a decrease in intracellular calcium influx through voltage-gated calcium channels rather than via adenylyl cyclase inhibition. Stimulation of dopamine D2 receptors in the nigrostriatal pathway leads to improvements in coordinated muscle activity in those with movement disorders. | |
| Record name | Bromocriptine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01200 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
25614-03-3 | |
| Record name | (+)-Bromocriptine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=25614-03-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Bromocriptine [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0025614033 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bromocriptine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01200 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bromocriptine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1022687 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Bromocriptine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.042.829 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | BROMOCRIPTINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3A64E3G5ZO | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Bromocriptine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015331 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
215-218 | |
| Record name | Bromocriptine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01200 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![(2S)-1-[(2S)-2-[[5-(2,3-dihydro-1-benzofuran-2-yl)-1-ethoxy-1-oxopentan-2-yl]amino]propanoyl]pyrrolidine-2-carboxylic acid](/img/structure/B1667802.png)
![6,6-Dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,3,5-triazine-2,4-diamine hydrochloride](/img/structure/B1667809.png)