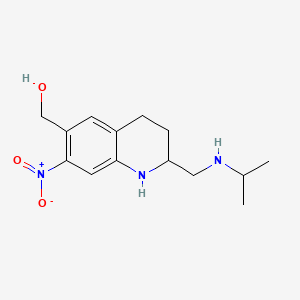
Oxamniquine
Übersicht
Beschreibung
Oxamniquine is a medication primarily used to treat schistosomiasis caused by Schistosoma mansoni . It is an anthelmintic agent that works by causing paralysis of the parasitic worms, leading to their detachment from the mesenteric veins and subsequent death . This compound is known for its effectiveness as a single-dose treatment and is particularly valuable in regions where schistosomiasis is endemic .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen: Die Synthese von Oxamniquin beginnt mit einem Molekül, das eine Chinolinstruktur enthält. Der Prozess umfasst mehrere Schlüsselreaktionen, darunter Substitution und Reduktion . Zunächst durchläuft das Ausgangsmolekül eine Substitutionsreaktion, die durch Natriumcarbonat aktiviert wird, um eine Zwischenstruktur zu bilden. Diese Zwischenverbindung unterliegt dann einer bimolekularen nukleophilen Substitution (SN2)-Reaktion, gefolgt von einer Hydrierung mit Nickel als Katalysator . Die letzten Schritte umfassen eine Nitrierung mit konzentrierter Salpetersäure und Schwefelsäure, gefolgt von einer mikrobiellen Oxidation, um Oxamniquin zu erhalten .
Industrielle Produktionsverfahren: Die industrielle Produktion von Oxamniquin folgt ähnlichen Synthesewegen, jedoch in größerem Maßstab. Der Prozess ist auf Ausbeute und Reinheit optimiert, um sicherzustellen, dass das Endprodukt pharmazeutische Standards erfüllt. Der Einsatz von automatisierten Reaktoren und strengen Qualitätskontrollmaßnahmen ist für die industrielle Synthese von Oxamniquin von entscheidender Bedeutung .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Oxamniquin unterliegt verschiedenen chemischen Reaktionen, darunter:
Substitutionsreaktionen: Dies beinhaltet den Austausch von funktionellen Gruppen innerhalb des Moleküls.
Reduktionsreaktionen: Zum Beispiel der Hydrierungsschritt in seiner Synthese.
Nitrierungsreaktionen: Einführung von Nitrogruppen unter Verwendung von Salpetersäure und Schwefelsäure.
Häufige Reagenzien und Bedingungen:
Natriumcarbonat: Wird in der anfänglichen Substitutionsreaktion verwendet.
Nickelkatalysator: Wird im Hydrierungsschritt eingesetzt.
Salpetersäure und Schwefelsäure: Wird für die Nitrierung verwendet.
Hauptsächlich gebildete Produkte: Das Hauptprodukt, das aus diesen Reaktionen gebildet wird, ist Oxamniquin selbst, wobei Zwischenverbindungen durch nachfolgende Schritte umgewandelt werden .
Wissenschaftliche Forschungsanwendungen
Oxamniquine is an antihelminthic drug primarily used in the treatment of schistosomiasis, a parasitic disease caused by blood flukes (schistosomes) . It is particularly effective against Schistosoma mansoni, one of the major species responsible for this disease .
Efficacy Against Schistosomiasis
This compound has been shown to be highly effective against S. mansoni . However, its effectiveness varies depending on the specific diagnostic methods used . Stool examinations have shown high cure rates with this compound, but when more sensitive methods like quantitative oogram by rectal biopsy are used, the cure rates can drop . Praziquantel, another drug used to treat schistosomiasis, maintains high cure rates regardless of the diagnostic method used .
A study comparing this compound and praziquantel found that stool examinations showed cure rates of 90.3% for this compound and 100% for praziquantel . However, when the oogram method was used, the cure rate for this compound dropped to 42.4%, while praziquantel remained high at 96.1% .
| Drug | Cure Rate (Stool Examination) | Cure Rate (Oogram Method) |
|---|---|---|
| This compound | 90.3% | 42.4% |
| Praziquantel | 100% | 96.1% |
Re-engineering this compound
Researchers have been working on re-engineering this compound to develop new treatments that can prevent drug resistance . Drug resistance occurs when the parasitic worm gains mutations and no longer responds to treatment . Derivatives of this compound have shown efficacy against all three schistosomiasis species (S. mansoni, S. haematobium, and S. japonicum) in vitro .
Structural data from collaborative studies have helped identify points that could be restructured to allow this compound to act differently in the body . This iterative process has led to the identification of derivatives that kill S. haematobium and S. japonicum .
This compound Derivatives
Several this compound derivatives have shown promising antischistosomal activity . These derivatives, including ferrocene-, ruthenocene-, and benzene-containing compounds, have demonstrated in vitro death of S. mansoni and S. haematobium adult worms . In vivo studies have shown worm burden reductions of 76 to 93% against adult S. mansoni .
Use in Conjunction with Other Drugs
Given the concerns about drug resistance, researchers are exploring the use of this compound in conjunction with other drugs like praziquantel . The goal is to design an this compound derivative that is effective against all major human schistosome species and can be used with praziquantel to combat emerging resistance and improve overall treatment efficacy .
Reduction of Morbidity
Even though this compound may not cure all patients in areas endemic for S. mansoni, its use has led to a marked reduction in disease morbidity, with a significant reduction in hepatosplenic forms of the disease in Brazil .
Rifaximin and Hepatosplenic Schistosomiasis
Wirkmechanismus
Oxamniquine exerts its effects by targeting the nucleic acid metabolism of Schistosoma mansoni . The drug is activated by a schistosome sulfotransferase enzyme, which converts this compound into an ester (likely acetate, phosphate, or sulfate) . This ester then dissociates, producing an electrophilic reactant capable of alkylating schistosome DNA, leading to the paralysis and death of the worms .
Vergleich Mit ähnlichen Verbindungen
Praziquantel: Another anthelmintic used to treat schistosomiasis, but with a broader spectrum of activity against various Schistosoma species.
Lucanthone: An older drug used for schistosomiasis with more side effects and less efficacy compared to oxamniquine.
Uniqueness of this compound: this compound is unique in its specific activity against Schistosoma mansoni and its ability to cause paralysis of the worms, leading to their detachment and death . Unlike praziquantel, this compound is not effective against other Schistosoma species, making it a more targeted treatment option .
Biologische Aktivität
Oxamniquine (OXA) is an antischistosomal drug that has been utilized primarily for the treatment of schistosomiasis, particularly against Schistosoma mansoni. This compound has garnered attention due to its unique mechanism of action and the potential for developing derivatives that enhance its efficacy against other schistosome species.
This compound is a prodrug that requires enzymatic activation to exert its biological effects. The activation occurs through a sulfotransferase enzyme present in S. mansoni, which converts OXA into a reactive alkylating agent. This active form then binds covalently to DNA and other macromolecules within the parasite, leading to cell death. The specific enzyme responsible for this activation has been identified as SmSULT-OR, a sulfotransferase that is absent in resistant schistosome strains .
In Vitro and In Vivo Studies
Recent studies have demonstrated the biological activity of OXA and its derivatives against various schistosome species. Notably, derivatives such as Fc-CH2-OXA and Rc-CH2-OXA have shown promising results in vitro against S. mansoni and S. haematobium. The activity of these compounds was assessed by measuring their half-maximal inhibitory concentration (IC50) values, which indicate the potency of the drug.
Table 1: In Vitro Activity of this compound Derivatives
| Compound | IC50 (µM) against S. mansoni | IC50 (µM) against S. haematobium | IC50 (µM) against S. japonicum |
|---|---|---|---|
| This compound | Not effective | Not effective | Not effective |
| Fc-CH2-OXA | 22.6 | 100 | 100 |
| Rc-CH2-OXA | 100 | 15.5 | Not tested |
| Ph-CH2-OXA | 100 | 100 | Not tested |
The results indicate that while OXA itself is ineffective against S. haematobium and S. japonicum, its derivatives exhibit significant activity, particularly Rc-CH2-OXA, which has a notably low IC50 value against S. haematobium .
Case Studies
A study conducted by Pasche et al. highlighted the importance of protein binding in drug efficacy. It was observed that the presence of albumin reduced the activity of OXA derivatives, emphasizing the need for further modifications to enhance bioavailability and therapeutic effectiveness . Additionally, another investigation focused on developing new derivatives through an iterative process involving soaking these compounds into sulfotransferase crystals, leading to the identification of two promising candidates: CIDD-0072229 and CIDD-149830, which demonstrated efficacy against both S. haematobium and S. japonicum .
Eigenschaften
IUPAC Name |
[7-nitro-2-[(propan-2-ylamino)methyl]-1,2,3,4-tetrahydroquinolin-6-yl]methanol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H21N3O3/c1-9(2)15-7-12-4-3-10-5-11(8-18)14(17(19)20)6-13(10)16-12/h5-6,9,12,15-16,18H,3-4,7-8H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XCGYUJZMCCFSRP-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NCC1CCC2=CC(=C(C=C2N1)[N+](=O)[O-])CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H21N3O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3023398 | |
| Record name | Oxamniquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3023398 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
279.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Oxamniquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015228 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1 part in about 3300 parts of water at 27 °C, 1.24e-01 g/L | |
| Record name | Oxamniquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01096 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | OXAMNIQUINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6510 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxamniquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015228 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Oxamniquine may associate with an irreversible inhibition of the nucleic acid metabolism of the parasites. A hypothesis has been put forth that the drug is activated by a single step, in which a schistosome sulfotransferase enzyme converts oxamniquine into an ester (probably acetate, phosphate, or sulfate). Subsequently, the ester spontaneously dissociates, the resulting electrophilic reactant is capable of alkylation of schistosome DNA., Causes the worms to be dislodged from their usual site of residence in the mesenteric veins to the liver where they are retained and subsequently killed by host tissue reactions (eg, phagocytosis). The dislodgment of worms appears to result principally from contraction and paralysis of their musculature and subsequent immobilization of their suckers, which causes the worms to detach from the blood vessel wall, thereby allowing passive dislodgement by normal blood flow., Hycanthone-sensitive and hycanthone-resistant schistosomes (which are also sensitive and resistant to oxamniquine) were exposed in vitro to tritium-labelled oxamniquine. The initial uptake of the drug into the schistosomes was essentially the same for the 2 strains. The homogenate of worms incubated with tritiated oxamniquine was fractionated and a purified DNA fraction was obtained by ethanol precipitation, RNAase and protease digestion, repeated phenolchloroform extractions, cesium chloride gradient centrifugation and extensive dialysis. The DNA fraction from sensitive worms contained radioactive oxamniquine at a level corresponding to about 1 drug molecule per 50,000 base pairs, while the DNA from resistant worms contained essentially no drug. The results support the hypothesis that oxamniquine, like hycanthone, exerts its activity by alkylating macromolecules of sensitive schistosomes. | |
| Record name | Oxamniquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01096 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | OXAMNIQUINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6510 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Pale yellow crystals from isopropanol, Yellow-orange, crystalline solid | |
CAS No. |
21738-42-1, 40247-39-0, 119678-90-9 | |
| Record name | Oxamniquine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=21738-42-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Oxamniquine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0021738421 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Oxamniquine, (+)- | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0040247390 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Oxamniquine, (-)- | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0119678909 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Oxamniquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01096 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | oxamniquine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=352888 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Oxamniquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3023398 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Oxamniquine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.040.491 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | OXAMNIQUINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0O977R722D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | OXAMNIQUINE, (-)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/00BCY677OT | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | OXAMNIQUINE, (+)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/7GIJ138H3K | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | OXAMNIQUINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6510 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxamniquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015228 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
147-149 °C, 147 - 149 °C | |
| Record name | Oxamniquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01096 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | OXAMNIQUINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6510 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxamniquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015228 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.
















