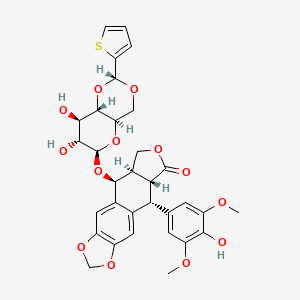
Teniposide
Übersicht
Beschreibung
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Teniposide is a semisynthetic derivative of podophyllotoxin. The synthesis involves the glycosylation of podophyllotoxin with a D-glucose derivative . The reaction conditions typically include the use of acidic or basic catalysts to facilitate the glycosylation process.
Industrial Production Methods: Industrial production of this compound involves large-scale extraction of podophyllotoxin from the rhizomes of the wild mandrake (Podophyllum peltatum), followed by chemical modification to produce this compound . The process is optimized to ensure high yield and purity of the final product.
Analyse Chemischer Reaktionen
Arten von Reaktionen: Teniposide unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann oxidiert werden, um reaktive Zwischenprodukte zu bilden.
Reduktion: Reduktionsreaktionen können die funktionellen Gruppen an this compound verändern.
Substitution: Substitutionsreaktionen können an bestimmten Stellen im this compound-Molekül auftreten.
Häufige Reagenzien und Bedingungen:
Oxidationsmittel: Wasserstoffperoxid, Kaliumpermanganat.
Reduktionsmittel: Natriumborhydrid, Lithiumaluminiumhydrid.
Substitutionsreagenzien: Halogene, Nukleophile.
Hauptprodukte: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, hängen von den verwendeten Reagenzien und Bedingungen ab. Beispielsweise kann Oxidation zur Bildung von Chinonen führen, während Reduktion Alkohole oder Amine ergeben kann.
Wissenschaftliche Forschungsanwendungen
Teniposide hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
Chemie: Als Modellverbindung verwendet, um die Mechanismen der Topoisomerase-II-Hemmung zu untersuchen.
Biologie: Untersucht wegen seiner Auswirkungen auf die Zellzyklusregulation und Apoptose.
Industrie: In der Entwicklung neuer Chemotherapeutika und Medikamententrägersysteme verwendet.
5. Wirkmechanismus
This compound übt seine Wirkung aus, indem es das Enzym Topoisomerase II hemmt, das für die DNA-Replikation und Zellteilung unerlässlich ist . Durch die Bildung eines Komplexes mit Topoisomerase II und DNA induziert this compound Brüche in doppelsträngiger DNA und verhindert die Reparatur, was zum Zelltod führt . Dieser Mechanismus betrifft hauptsächlich Zellen in der G2- und S-Phase des Zellzyklus .
Ähnliche Verbindungen:
Etoposide: Ein weiteres Podophyllotoxin-Derivat mit ähnlichem Wirkmechanismus.
Podophyllotoxin: Die Stammverbindung, von der this compound abgeleitet ist.
Vergleich:
Etoposide vs. This compound: Beide Verbindungen hemmen die Topoisomerase II, aber this compound hat eine stärkere Penetration des zentralen Nervensystems und ist lipophiler.
Podophyllotoxin vs. This compound: Während Podophyllotoxin der natürliche Vorläufer ist, wird this compound chemisch modifiziert, um seine therapeutische Wirksamkeit zu verbessern und die Toxizität zu reduzieren.
Die einzigartigen Eigenschaften von this compound, wie seine höhere Lipophilie und größere Penetration des zentralen Nervensystems, machen es zu einem wertvollen Chemotherapeutikum mit eindeutigen Vorteilen gegenüber ähnlichen Verbindungen .
Wirkmechanismus
Teniposide exerts its effects by inhibiting the enzyme topoisomerase II, which is essential for DNA replication and cell division . By forming a complex with topoisomerase II and DNA, this compound induces breaks in double-stranded DNA and prevents repair, leading to cell death . This mechanism primarily affects cells in the G2 and S phases of the cell cycle .
Vergleich Mit ähnlichen Verbindungen
Etoposide: Another podophyllotoxin derivative with a similar mechanism of action.
Podophyllotoxin: The parent compound from which teniposide is derived.
Comparison:
Etoposide vs. This compound: Both compounds inhibit topoisomerase II, but this compound has greater central nervous system penetrance and is more lipophilic.
Podophyllotoxin vs. This compound: While podophyllotoxin is the natural precursor, this compound is chemically modified to improve its therapeutic efficacy and reduce toxicity.
This compound’s unique properties, such as its higher lipophilicity and greater central nervous system penetrance, make it a valuable chemotherapeutic agent with distinct advantages over similar compounds .
Biologische Aktivität
Teniposide, a semisynthetic derivative of podophyllotoxin, is primarily recognized for its antitumor properties, particularly in the treatment of various cancers such as leukemia and gliomas. This article delves into the biological activity of this compound, highlighting its mechanisms of action, clinical applications, and recent research findings.
This compound exerts its cytotoxic effects primarily by inhibiting DNA topoisomerase II, an enzyme crucial for DNA replication and transcription. This inhibition leads to the formation of DNA breaks and ultimately results in cell death. The drug is phase-specific, acting predominantly during the late S or early G2 phases of the cell cycle .
Key Mechanisms:
- Inhibition of Topoisomerase II : Stabilizes the topoisomerase II-DNA complex, leading to double-stranded DNA breaks.
- Cell Cycle Arrest : Prevents mitosis by interfering with DNA synthesis and repair processes .
Clinical Applications
This compound has been employed in various clinical settings, particularly in pediatric oncology and for treating high-grade gliomas. Below are notable case studies and clinical findings:
Case Study: Pediatric Acute Lymphoblastic Leukemia (ALL)
A study indicated that this compound is effective when used in combination chemotherapy regimens for treating childhood ALL. It has shown promising results in achieving remission when combined with other agents like ara-C .
Case Series: Intra-Arterial Delivery in Gliomas
A recent study evaluated the safety and efficacy of superselective intra-arterial delivery of this compound in patients with high-grade gliomas. Twelve patients received multiple cycles of treatment after blood-brain barrier disruption. The results showed:
- Complete Response : 2 patients (16.7%)
- Partial Response : 4 patients (33.3%)
- Disease Control Rate : 83.3% overall .
Recent Research Findings
Recent studies have uncovered novel roles for this compound beyond its traditional applications:
Targeting Epithelial-Mesenchymal Transition (EMT)
A groundbreaking study highlighted this compound's ability to modulate EMT in breast cancer cells by downregulating ZEB2, a key transcription factor involved in metastasis. The study demonstrated that low doses of this compound could significantly reduce mesenchymal-like characteristics and inhibit pulmonary colonization of cancer cells .
Summary of Biological Activities
Q & A
Basic Research Questions
Q. What are the validated molecular mechanisms of Teniposide's action in cancer therapy, and what experimental methodologies are used to confirm these targets?
this compound primarily inhibits topoisomerase II, stabilizing DNA-topoisomerase II complexes and inducing double-strand breaks. Researchers validate this mechanism via in vitro enzyme inhibition assays (measuring IC50 values) and in vivo tumor xenograft models to assess DNA damage markers (e.g., γ-H2AX foci) . Dose-response curves and comparative studies with other topoisomerase inhibitors (e.g., etoposide) are critical for specificity validation.
Q. What standardized protocols are recommended for this compound administration in preclinical studies, and how are pharmacokinetic parameters optimized?
Preclinical protocols typically use intraperitoneal or intravenous administration in murine models, with dosages calibrated to body surface area (e.g., 10–15 mg/kg in mice). Pharmacokinetic optimization involves HPLC analysis of plasma concentrations to determine clearance rates and bioavailability. Researchers must account for vehicle composition (e.g., Cremophor EL) to avoid confounding toxicity .
Q. Which experimental models are most appropriate for studying this compound resistance, and what biomarkers are indicative of resistance mechanisms?
Cell lines with acquired resistance (e.g., leukemia CCRF-CEM subclones) are used to study ATP-binding cassette (ABC) transporter upregulation (e.g., P-glycoprotein). Flow cytometry for efflux pump activity and RNA-seq for gene expression profiling are standard methods. Resistance biomarkers include reduced intracellular drug accumulation and overexpression of MDR1 .
Advanced Research Questions
Q. How can contradictory data on this compound’s efficacy in solid tumors versus hematological malignancies be systematically addressed?
Contradictions arise from tumor microenvironment differences (e.g., blood-brain barrier penetration in gliomas). Researchers should perform meta-analyses stratified by cancer type, adjusting for covariates like prior therapies and drug combinations. In silico modeling of tissue-specific pharmacokinetics and patient-derived organoid screens can clarify context-dependent efficacy .
Q. What methodologies are effective in identifying and mitigating hypersensitivity reactions to this compound in high-risk populations?
Hypersensitivity incidence is higher in neuroblastoma and brain tumor patients (6.5% overall, 82% in these cohorts). Prophylactic strategies include premedication with antihistamines/corticosteroids and graded rechallenge protocols. Immunoassays (e.g., IgE levels) and lymphocyte activation tests help identify at-risk patients .
Q. How can researchers optimize this compound’s synergistic potential with other antineoplastic agents while minimizing overlapping toxicities?
Combinatorial screens (e.g., Chou-Talalay synergy assays) identify synergistic pairs (e.g., this compound + cisplatin). Pharmacodynamic modeling predicts additive vs. antagonistic effects. Toxicity mitigation requires staggered dosing schedules and real-time monitoring of hematological parameters (e.g., neutrophil counts) .
Q. Methodological Guidance
Q. What statistical approaches are recommended for analyzing this compound’s dose-response relationships in heterogeneous tumor samples?
Non-linear mixed-effects models (NLMEM) account for inter-tumor variability. Bayesian hierarchical models integrate prior pharmacokinetic data to refine posterior dose estimates. Survival analyses (Cox proportional hazards) should stratify by genetic biomarkers (e.g., TP53 status) .
Q. How should researchers design longitudinal studies to assess this compound’s long-term genomic instability risks?
Longitudinal cohorts require periodic whole-genome sequencing to track clonal evolution and secondary malignancies. Comparative genomic hybridization (CGH) and micronucleus assays quantify chromosomal aberrations. Ethical frameworks must address incidental findings (e.g., germline mutations) .
Q. Data Interpretation and Reporting
Q. What criteria should guide the inclusion/exclusion of outlier data in this compound efficacy trials?
Outliers are assessed via Grubbs’ test or Dixon’s Q-test. Justification for exclusion requires documentation of technical errors (e.g., dosing miscalculations) or biological anomalies (e.g., undiagnosed comorbidities). Sensitivity analyses must report outcomes with and without outliers .
Q. How can researchers reconcile discrepancies between in vitro cytotoxicity assays and in vivo tumor regression results?
Discrepancies often stem from tumor stroma interactions or immune modulation. Orthotopic models and co-cultures with fibroblasts/immune cells improve translatability. Pharmacokinetic-pharmacodynamic (PK-PD) modeling bridges in vitro IC50 values to in vivo effective doses .
Eigenschaften
IUPAC Name |
(5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-thiophen-2-yl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H32O13S/c1-37-19-6-13(7-20(38-2)25(19)33)23-14-8-17-18(42-12-41-17)9-15(14)28(16-10-39-30(36)24(16)23)44-32-27(35)26(34)29-21(43-32)11-40-31(45-29)22-4-3-5-46-22/h3-9,16,21,23-24,26-29,31-35H,10-12H2,1-2H3/t16-,21+,23+,24-,26+,27+,28+,29+,31+,32-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NRUKOCRGYNPUPR-QBPJDGROSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=CC(=CC(=C1O)OC)C2C3C(COC3=O)C(C4=CC5=C(C=C24)OCO5)OC6C(C(C7C(O6)COC(O7)C8=CC=CS8)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC1=CC(=CC(=C1O)OC)[C@H]2[C@@H]3[C@H](COC3=O)[C@@H](C4=CC5=C(C=C24)OCO5)O[C@H]6[C@@H]([C@H]([C@H]7[C@H](O6)CO[C@H](O7)C8=CC=CS8)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H32O13S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8023638 | |
| Record name | Teniposide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023638 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
656.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Practically insoluble in water., In water, 5.9 mg/L at 25 °C /Estimated/ | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Vapor Pressure |
6.8X10-26 mm Hg at 25 °C /Estimated/ | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
The mechanism of action appears to be related to the inhibition of type II topoisomerase activity since teniposide does not intercalate into DNA or bind strongly to DNA. Teniposide binds to and inhibits DNA topoisomerase II. The cytotoxic effects of teniposide are related to the relative number of double-stranded DNA breaks produced in cells, which are a reflection of the stabilization of a topoisomerase II-DNA intermediate., It is an inhibitor of DNA topoisomerase II enzymes: Teniposide is a DNA topoisomerase II poison that has been shown to promote DNA cleavage, with a strong preference for a C or T at position -1. Most of the mutational events reported in mammalian cells, including point mutations, chromosomal deletions and exchanges and aneuploidy, can be explained by this activity. Teniposide does not inhibit bacterial topoisomerases and may not mutate bacterial cells by the same mechanism as mammalian cells. Unlike many other DNA topoisomerase II poisons, teniposide does not bind to DNA, either covalently or by intercalation. Instead, it appears to interact directly with the DNA topoisomerase II enzyme., ... The drug appears to produce its cytotoxic effects by damaging DNA and thereby inhibiting or altering DNA synthesis. Teniposide has been shown to induce single-stranded DNA breaks; the drug also induces double-stranded DNA breaks and DNA-protein cross links. ... Teniposide appears to be cell cycle specific, inducing G2-phase arrest and preferentially killing cells in the G2 and late S phases. | |
| Record name | Teniposide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00444 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
... The 50-mg intravenous preparation typically also contains benzyl alcohol (0.15 g), N,N-dimethylacetamide (0.3 g), polyethoxylated castor oil (2.5 g), maleic acid to a pH of 5.1 and absolute ethanol to 5 mL. | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from absolute ethanol | |
CAS No. |
29767-20-2 | |
| Record name | Teniposide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=29767-20-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Teniposide [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0029767202 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Teniposide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00444 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Teniposide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023638 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Teniposide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.045.286 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TENIPOSIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/957E6438QA | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
242-246 °C | |
| Record name | Teniposide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00444 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | TENIPOSIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6546 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.















