AMD-070
Descripción general
Descripción
Mavorixafor es un antagonista selectivo del receptor 4 de quimiocina C-X-C (CXCR4) biodisponible por vía oral. Se desarrolla principalmente para el tratamiento del síndrome de verrugas, hipogammaglobulinemia, infecciones y mielocatexis (WHIM), un trastorno de inmunodeficiencia primaria raro. Mavorixafor también se está investigando por su potencial en el tratamiento de varios cánceres, incluido el melanoma, y otros trastornos neutropénicos crónicos .
Aplicaciones Científicas De Investigación
WHIM Syndrome
WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis) is a rare immunodeficiency caused by gain-of-function mutations in the CXCR4 gene. Mavorixafor has been shown to significantly improve clinical outcomes in patients with WHIM syndrome:
- Clinical Trial Results : In a Phase 3 randomized trial, mavorixafor demonstrated a 60% reduction in annualized infection rates compared to placebo (1.7 vs. 4.2 infections) and improved absolute neutrophil counts (ANC) .
- Mechanism : By antagonizing CXCR4, mavorixafor facilitates the release of neutrophils into circulation, thereby enhancing the immune response .
Chronic Neutropenia
Mavorixafor is also being investigated for its efficacy in treating chronic neutropenia (CN), a condition characterized by persistently low levels of neutrophils:
- Phase 2 Trial Findings : A six-month study indicated that mavorixafor treatment led to durable increases in ANC and allowed for significant reductions in granulocyte colony-stimulating factor (G-CSF) dosing without compromising ANC levels .
- Safety Profile : The treatment was well tolerated with mild to moderate side effects predominantly gastrointestinal in nature .
Table 1: Summary of Clinical Trials Involving Mavorixafor
Cancer Treatment
Mavorixafor's role extends into oncology, where it is being explored for its potential to enhance the efficacy of existing cancer therapies:
- Immune Modulation : In a study involving melanoma patients, mavorixafor was administered alongside pembrolizumab to evaluate its effect on immune cell infiltration into tumors. Results suggested improved immune profiles within the tumor microenvironment .
- Potential Indications : Research indicates that mavorixafor could be beneficial for various malignancies including hematologic cancers and solid tumors due to its ability to mobilize immune cells effectively .
Mecanismo De Acción
Mavorixafor ejerce sus efectos uniéndose selectivamente al receptor CXCR4, bloqueando la unión de su ligando natural, el ligando 12 de la quimiocina C-X-C (también conocido como factor derivado del estroma-1). Esta inhibición previene la activación de las vías de señalización CXCR4, que están involucradas en el tráfico de células inmunitarias, la localización de células madre hematopoyéticas y la progresión tumoral. Al modular estas vías, mavorixafor mejora la infiltración y activación de células inmunitarias en el microambiente tumoral, lo que lleva a respuestas antitumorales mejoradas .
Análisis Bioquímico
Biochemical Properties
AMD-070 interacts with the CXCR4 chemokine receptor, acting as an antagonist . It binds to the transmembrane regions of the coreceptor, blocking the interaction of the CD4-gp120 complex with the ECL2 domain of the CXCR4 coreceptor .
Cellular Effects
This compound has shown to significantly suppress the anchorage-dependent growth, migration, and matrigel invasion of B88-SDF-1 cells . It also inhibits the replication of T-tropic HIV-1 (NL4.3 strain) in MT-4 cells and PBMCs .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to the transmembrane regions of the CXCR4 coreceptor, thereby preventing CXCR4-mediated viral entry of T-cell tropic synctium-inducing HIV . This action blocks the interaction of the CD4-gp120 complex with the ECL2 domain of the CXCR4 coreceptor .
Dosage Effects in Animal Models
It has been shown that this compound (2 mg/kg, p.o.) significantly reduces the number of metastatic lung nodules in mice .
Metabolic Pathways
This compound is primarily metabolized by cytochrome P450 (CYP) 3A4 and to a lesser extent, CYP2D6 . It is also a substrate of P-glycoprotein (P-gp) .
Transport and Distribution
It is known that this compound is a substrate of P-glycoprotein (P-gp), which plays a role in drug transport .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
La síntesis de mavorixafor implica múltiples pasos, comenzando con materiales de partida disponibles comercialmenteLas condiciones de reacción suelen implicar el uso de disolventes orgánicos, catalizadores y temperaturas controladas para garantizar un alto rendimiento y pureza .
Métodos de producción industrial
La producción industrial de mavorixafor sigue una ruta sintética similar, pero está optimizada para la fabricación a gran escala. Esto incluye el uso de reactores de flujo continuo, sistemas de purificación automatizados y estrictas medidas de control de calidad para garantizar la coherencia y el cumplimiento de las normas reglamentarias .
Análisis De Reacciones Químicas
Tipos de reacciones
Mavorixafor experimenta varias reacciones químicas, que incluyen:
Oxidación: Mavorixafor se puede oxidar en condiciones específicas para formar derivados oxidados.
Reducción: Las reacciones de reducción se pueden usar para modificar la porción de quinolina.
Sustitución: Las reacciones de sustitución se emplean para introducir diferentes grupos funcionales en el núcleo de benzimidazol.
Reactivos y condiciones comunes
Oxidación: Los agentes oxidantes comunes incluyen peróxido de hidrógeno y permanganato de potasio.
Reducción: Se utilizan agentes reductores como borohidruro de sodio e hidruro de aluminio y litio.
Sustitución: Se utilizan reactivos como haluros de alquilo y haluros de arilo para reacciones de sustitución.
Productos principales
Los principales productos formados a partir de estas reacciones incluyen varios derivados de mavorixafor con grupos funcionales modificados, que se pueden investigar más a fondo por sus propiedades farmacológicas .
Comparación Con Compuestos Similares
Compuestos similares
Plerixafor: Otro antagonista de CXCR4 utilizado para la movilización de células madre en pacientes con linfoma no Hodgkin y mieloma múltiple.
Unicidad
Mavorixafor es único en su biodisponibilidad oral y selectividad para el receptor CXCR4. A diferencia de plerixafor y AMD3100, que se administran mediante inyección, mavorixafor se puede tomar por vía oral, lo que lo hace más conveniente para los pacientes. Además, su selectividad para CXCR4 reduce la probabilidad de efectos fuera del objetivo, mejorando su perfil de seguridad .
Actividad Biológica
Mavorixafor is an orally bioavailable, selective antagonist of the CXCR4 chemokine receptor, primarily developed for treating conditions such as WHIM syndrome and chronic neutropenia. Its biological activity is characterized by the modulation of immune cell trafficking, enhancing the mobilization of neutrophils and lymphocytes from the bone marrow into circulation. This article synthesizes findings from various studies to elucidate the biological activity of mavorixafor, focusing on its mechanisms, clinical efficacy, and safety profile.
Mavorixafor functions by inhibiting the CXCR4 receptor, which plays a critical role in the retention of immune cells in the bone marrow. By blocking this receptor, mavorixafor facilitates the release of white blood cells into the bloodstream, thereby increasing their availability for immune responses.
Key Mechanisms:
- Inhibition of CXCR4 : Reduces retention of immune cells in bone marrow.
- Increased Immune Cell Mobilization : Enhances circulating neutrophil and lymphocyte counts.
- Modulation of Tumor Microenvironment (TME) : Increases CD8+ T-cell infiltration in tumors, potentially improving responses to immunotherapies.
Phase Ib Study in Melanoma
A biomarker-driven Phase Ib study (NCT02823405) evaluated mavorixafor's effects on immune cell profiles within the TME of melanoma patients.
| Parameter | Results |
|---|---|
| CD8+ T-cell Infiltration | Increased significantly after 3 weeks of monotherapy |
| Granzyme B Signal | Enhanced expression observed |
| Cytokines (CXCL9, CXCL10) | Elevated levels when combined with pembrolizumab |
| Adverse Events | Diarrhea, fatigue, rash (all ≤ grade 3) |
The study concluded that mavorixafor enhances immune cell infiltration and activation within tumors, suggesting its potential to improve outcomes for patients unresponsive to checkpoint inhibitors .
Phase II Study in WHIM Syndrome
A Phase II open-label study assessed mavorixafor's safety and efficacy in patients with WHIM syndrome.
| Parameter | Results |
|---|---|
| Neutrophil Count (ANC) | Statistically significant increases; maintained >500 cells/µL for 12.6 hours at doses ≥300 mg/day |
| Lymphocyte Count (ALC) | Maintained >1000 cells/µL for up to 16.9 hours |
| Safety Profile | Well-tolerated; no serious adverse events reported |
The study demonstrated that mavorixafor effectively mobilizes neutrophils and lymphocytes, contributing to improved clinical outcomes in patients with this rare immunodeficiency .
Phase III Trial Outcomes
In a Phase III trial involving patients with WHIM syndrome, mavorixafor was compared to placebo over 52 weeks.
| Parameter | Mavorixafor Group | Placebo Group |
|---|---|---|
| Neutrophil Count Increase | Significant increase observed | Not applicable |
| Infection Rate Reduction | Statistically significant decrease | Not applicable |
Adverse effects included low platelet counts and mild rashes but were generally manageable .
Case Studies
- Case Study 1 : A patient with WHIM syndrome treated with mavorixafor exhibited a two-fold increase in ANC after three months, leading to a marked reduction in infection frequency.
- Case Study 2 : In melanoma patients, combination therapy with mavorixafor and pembrolizumab resulted in enhanced tumor-infiltrating lymphocyte activity, correlating with improved overall survival metrics.
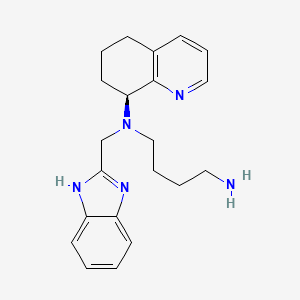
Propiedades
IUPAC Name |
N'-(1H-benzimidazol-2-ylmethyl)-N'-[(8S)-5,6,7,8-tetrahydroquinolin-8-yl]butane-1,4-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H27N5/c22-12-3-4-14-26(15-20-24-17-9-1-2-10-18(17)25-20)19-11-5-7-16-8-6-13-23-21(16)19/h1-2,6,8-10,13,19H,3-5,7,11-12,14-15,22H2,(H,24,25)/t19-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WVLHHLRVNDMIAR-IBGZPJMESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(C2=C(C1)C=CC=N2)N(CCCCN)CC3=NC4=CC=CC=C4N3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1C[C@@H](C2=C(C1)C=CC=N2)N(CCCCN)CC3=NC4=CC=CC=C4N3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H27N5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60971247 | |
| Record name | N~1~-[(1H-Benzimidazol-2-yl)methyl]-N~1~-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60971247 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
349.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Chemokine receptors expressed on the surface of immune cells are known to play a critical role in virus infection and transmission. CXCR4, and another chemokine receptor CCR5, are involved in HIV infection. The process of HIV entry begins with binding of the viral envelope glycoprotein to both the CD4 receptor and one of only two chemokine receptors, and ends with fusion of viral and cell membranes. Viral entry provides novel therapeutic targets against HIV. To date, at least 3 sub classes of HIV viral entry/fusion inhibitors have emerged: 1. CD4 binding or attachment - targets initial recognition and binding of the viral glycoprotein gp120 to the cell-surface CD4 antigen. 2. Chemokine co-receptor binding - targets binding of virus to the CCR5 or CXCR4 co-receptor. 3. Fusion Inhibition - targets the viral glycoprotein gp41 inhibiting the fusion of virus with the cell. Different strains of HIV prefer one receptor or the other, or may use either receptor to infect cells. * 35% of strains use both CXCR4 and CCR5 * 5% of strains are pure CXCR4 using * 60% of strains are pure CCR5 using * An infected individual may harbor different levels of both CXCR4 and CCR5 using virus * CXCR4 using virus independently predicts CD4 decline and HIV clinical progression and is associated with earlier mortality | |
| Record name | AMD-070 | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB05501 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
558447-26-0 | |
| Record name | Mavorixafor [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0558447260 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | AMD-070 | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB05501 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | N~1~-[(1H-Benzimidazol-2-yl)methyl]-N~1~-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60971247 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | MAVORIXAFOR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0G9LGB5O2W | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details






Synthesis routes and methods II
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.













