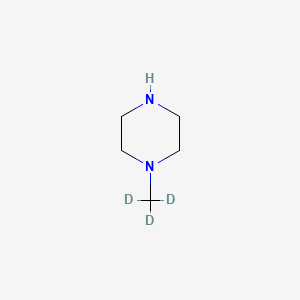
1-(Trideuteriomethyl)piperazine
Vue d'ensemble
Description
1-(Trideuteriomethyl)piperazine is a deuterated derivative of piperazine, a heterocyclic organic compound The deuterium atoms replace the hydrogen atoms in the methyl group, which can influence the compound’s physical and chemical properties
Méthodes De Préparation
Synthetic Routes and Reaction Conditions: 1-(Trideuteriomethyl)piperazine can be synthesized through various methods. One common approach involves the deuteration of 1-methylpiperazine using deuterium gas or deuterated reagents. The reaction typically requires a catalyst and specific conditions to ensure the incorporation of deuterium atoms.
Industrial Production Methods: Industrial production of this compound often involves large-scale deuteration processes. These processes are optimized for high yield and purity, utilizing advanced catalytic systems and controlled reaction environments to achieve the desired isotopic substitution.
Analyse Des Réactions Chimiques
Types of Reactions: 1-(Trideuteriomethyl)piperazine undergoes various chemical reactions, including:
Oxidation: The compound can be oxidized to form corresponding N-oxides.
Reduction: Reduction reactions can lead to the formation of deuterated amines.
Substitution: Nucleophilic substitution reactions can occur at the nitrogen atoms or the deuterated methyl group.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and peracids.
Reduction: Reducing agents such as lithium aluminum hydride or sodium borohydride are used.
Substitution: Halogenated compounds and strong bases are often employed in substitution reactions.
Major Products: The major products formed from these reactions depend on the specific reagents and conditions used. For example, oxidation can yield N-oxides, while reduction can produce deuterated amines.
Applications De Recherche Scientifique
Chemical Research Applications
Stable Isotope Labeling
1-(Trideuteriomethyl)piperazine serves as a stable isotope-labeled compound in analytical chemistry. It is particularly valuable in mass spectrometry, where it aids in the identification and quantification of substances by providing distinct mass signatures due to the presence of deuterium. This allows for improved accuracy in experiments involving complex mixtures.
Synthesis of Other Compounds
The compound is also used as a building block in the synthesis of other deuterated compounds. Its unique isotopic properties can enhance the stability and reactivity profiles of synthesized molecules, making it an essential intermediate in organic synthesis.
Biological Research Applications
Metabolic Pathway Studies
In biological research, this compound is employed to investigate metabolic pathways and enzyme kinetics. The incorporation of deuterium can influence the metabolic fate of compounds, allowing researchers to trace metabolic processes more effectively.
Pharmacokinetic Studies
The compound has been investigated for its potential use in drug development, particularly in pharmacokinetic studies. By using deuterated compounds, researchers can gain insights into drug metabolism and disposition without the confounding effects that non-deuterated analogs might introduce.
Medicinal Chemistry Applications
Drug Development
this compound has shown promise in the development of new pharmaceutical agents. Its unique isotopic labeling can alter pharmacokinetic properties, potentially leading to drugs with improved efficacy and reduced side effects. For example, deuterated drugs often exhibit altered metabolic pathways that can enhance their therapeutic profiles.
Case Study: Deuterated Drug Candidates
Several studies have highlighted the advantages of using deuterated compounds in drug development. A notable example includes the investigation of deuterated versions of existing drugs that demonstrated improved pharmacokinetics while maintaining similar pharmacodynamics. This approach not only enhances drug performance but also reduces the risk of adverse effects associated with standard formulations.
Industrial Applications
Quality Control Standards
In industrial settings, this compound is utilized as a reference standard for quality control processes. Its stable isotopic nature allows for precise calibration and validation in analytical methods used across various industries, including pharmaceuticals and biochemistry.
Data Table: Summary of Applications
| Application Area | Specific Use Case | Benefits |
|---|---|---|
| Chemical Research | Stable isotope labeling for mass spectrometry | Enhanced accuracy and sensitivity |
| Biological Research | Metabolic pathway tracing | Improved understanding of metabolism |
| Medicinal Chemistry | Drug development and pharmacokinetic studies | Potential for better drug profiles |
| Industrial Applications | Quality control standards | Precision in analytical methods |
Mécanisme D'action
The mechanism of action of 1-(Trideuteriomethyl)piperazine involves its interaction with molecular targets and pathways. The deuterium atoms can influence the compound’s binding affinity and metabolic stability. This can lead to altered pharmacokinetics and pharmacodynamics, making it a valuable tool in drug development and research.
Comparaison Avec Des Composés Similaires
1-(Trideuteriomethyl)piperazine can be compared with other deuterated piperazine derivatives and non-deuterated analogs:
Similar Compounds: 1-methylpiperazine, 1-(trideuteriomethyl)piperidine, and 1-(trideuteriomethyl)morpholine.
Uniqueness: The presence of deuterium atoms in this compound provides enhanced stability and altered metabolic pathways compared to its non-deuterated counterparts. This makes it particularly useful in applications requiring precise isotopic labeling and stability.
Activité Biologique
1-(Trideuteriomethyl)piperazine is a deuterated analog of piperazine, a cyclic amine that has garnered attention for its applications in medicinal chemistry and biological research. The incorporation of deuterium introduces unique isotopic properties that can significantly influence the compound's metabolic pathways and biological interactions. This article explores the biological activity of this compound, highlighting its mechanisms of action, therapeutic potential, and relevant case studies.
This compound has the molecular formula C₄H₁₀D₃N and a molecular weight of approximately 101.16 g/mol. The presence of deuterium alters the physicochemical properties of the compound, impacting its solubility, stability, and metabolic behavior compared to its non-deuterated counterparts.
Mechanisms of Biological Activity
The biological activity of this compound can be attributed to several mechanisms:
- Metabolic Pathway Studies : The unique isotopic labeling allows researchers to trace metabolic pathways more effectively. This is particularly useful in pharmacokinetic studies where understanding the metabolism of drugs is crucial for determining their efficacy and safety.
- Enzyme Interactions : Deuterated compounds often exhibit altered interactions with enzymes due to changes in kinetic isotope effects. This can lead to variations in reaction rates and pathways, making this compound a valuable tool in enzyme mechanism studies .
- Therapeutic Applications : Research indicates that deuterated compounds can have improved pharmacokinetic profiles, including reduced clearance rates and enhanced half-lives. These properties make them suitable candidates for therapeutic applications in various diseases, including neurological disorders .
Case Studies and Research Findings
Several studies have investigated the biological activity of this compound:
- Pharmacokinetics : A study demonstrated that deuterated analogs exhibit significantly slower metabolic clearance compared to their non-deuterated forms. This was evidenced by a 70% reduction in clearance rates for certain compounds when deuterium was substituted at specific positions .
- Neuropharmacological Effects : Research into PDE1 inhibitors has shown that compounds like this compound can enhance levels of cyclic nucleotides (cAMP/cGMP), which are crucial for neuronal signaling. This modulation has implications for treating psychiatric disorders such as depression and anxiety .
- Cancer Research : In oncology, the use of deuterated compounds has been linked to reduced toxicity and improved therapeutic indices. For instance, studies have indicated that deuterated versions of cytotoxic agents may offer enhanced selectivity towards cancer cells while minimizing damage to healthy tissues .
Data Table: Comparative Biological Activity
| Property/Activity | Non-Deuterated Piperazine | This compound |
|---|---|---|
| Molecular Weight | 86.12 g/mol | 101.16 g/mol |
| Clearance Rate | Standard | Reduced by ~70% |
| Half-Life | Standard | Increased |
| Enzyme Interaction | Standard | Altered due to isotope effect |
| Therapeutic Index | Variable | Improved |
Propriétés
IUPAC Name |
1-(trideuteriomethyl)piperazine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C5H12N2/c1-7-4-2-6-3-5-7/h6H,2-5H2,1H3/i1D3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PVOAHINGSUIXLS-FIBGUPNXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCNCC1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])N1CCNCC1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C5H12N2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
103.18 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Synthesis routes and methods I
Procedure details













Synthesis routes and methods II
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.













