Cytarabine
Vue d'ensemble
Description
Cytarabine, également connue sous le nom d’arabinoside de cytosine, est un médicament de chimiothérapie principalement utilisé pour traiter diverses formes de leucémie, notamment la leucémie aiguë myéloïde, la leucémie aiguë lymphoblastique et la leucémie myéloïde chronique. Il est également utilisé dans le traitement du lymphome non hodgkinien. La this compound est un analogue nucléosidique de la pyrimidine qui inhibe la synthèse de l’ADN, ce qui la rend efficace pour cibler les cellules cancéreuses qui se divisent rapidement .
Méthodes De Préparation
Voies de synthèse et conditions de réaction
La cytarabine est synthétisée à partir de la cytidine par une série de réactions chimiques. La méthode de préparation traditionnelle implique l’utilisation d’acide polyphosphorique pour synthétiser la this compound à partir de la cytidine. Les conditions de réaction comprennent généralement un chauffage et l’utilisation de solvants tels que l’eau et l’alcool. Le rendement de ce processus varie de 29 % à 40 % .
Méthodes de production industrielle
Dans les milieux industriels, la this compound est produite en grandes quantités en utilisant des voies de synthèse similaires, mais avec des conditions de réaction optimisées pour maximiser le rendement et la pureté. Le processus implique l’utilisation de techniques de purification avancées pour garantir que le produit final répond aux normes pharmaceutiques .
Analyse Des Réactions Chimiques
Hydrolysis and Deamination
Cytarabine undergoes pH-dependent hydrolysis and enzymatic deamination to form 1-β-D-arabinofuranosyluracil (ara-U) , its primary inactive metabolite.
Key Reactions:
- Deamination by cytidine deaminase :
This compound → ara-U (via NH₃ elimination) - Acid-catalyzed hydrolysis :
Degrades via cleavage of the glycosidic bond at pH < 3 . - Base-catalyzed degradation :
Forms uracil arabinoside under alkaline conditions (pH > 9) .
Table 1: Thermodynamic Parameters of this compound Degradation
| Parameter | Value (Citric Acid) | Value (Saline) | Source |
|---|---|---|---|
| ΔH (kJ/mol) | -5.42 | -20.40 | |
| Half-life (min) | 45.12 | 62.45 | |
| Activation Energy (kJ/mol) | 28.24 | 23.21 |
Phosphorylation and DNA Incorporation
This compound requires intracellular activation via phosphorylation to exert cytotoxic effects:
Metabolic Pathway:
- Phosphorylation by deoxycytidine kinase → This compound monophosphate (ara-CMP)
- Subsequent phosphorylation → This compound triphosphate (ara-CTP)
- Incorporation into DNA :
Table 2: Kinetic Effects of this compound on Model Okazaki Fragments
| Parameter | Control (OKA) | This compound-Substituted (ARAC) |
|---|---|---|
| Melting Temp (°C) | 46.8 | 42.4 |
| ΔG (kcal/mol at 37°C) | -4.1 | -2.9 |
| DNA Duplex Destabilization | None | Observed via ¹H NMR |
Interaction with Enzymatic Targets
This compound’s reactivity is governed by its inhibition of key enzymes:
Enzymatic Inhibition:
- DNA polymerase α/β : Direct competitive inhibition (Kᵢ = 0.1–1.0 μM)
- Ribonucleotide reductase : Reduces dCTP pools, enhancing ara-CTP incorporation
- Cytidine deaminase : Primary catabolic enzyme (Km = 0.2 mM)
Table 3: Stability in Clinical Formulations
| Condition | Degradation Rate (%) | Half-life |
|---|---|---|
| pH 7.4, 25°C | <5% over 7 days | 7 days |
| pH 5.0, 40°C | 15% over 24 hours | 6.8 hours |
| Light exposure | 10% over 48 hours | 48 hours |
Photodegradation and Oxidative Pathways
Advanced oxidation processes (AOPs) degrade this compound in wastewater:
- UV/H₂O₂ systems : Achieve >95% degradation via hydroxyl radical (·OH) attack
- Fenton-like reactions : Fe³⁺/UV systems yield 80% degradation in 60 min
- Primary degradation products : Arabinofuranosyluracil, cytosine, and arabinose
Solid-State Reactivity
This compound exhibits distinct hydrogen-bonding patterns influencing stability:
- Intramolecular H-bonds : O2’–H···O5’ stabilizes arabinose conformation
- Crystal packing : NH···O interactions with Gln97/Asp133 in dCK complexes
Compatibility with Excipients
This compound demonstrates variable stability in parenteral admixtures:
| Excipient | Compatibility | Observations |
|---|---|---|
| 0.9% NaCl | Stable | No precipitation over 7 days |
| 5% Dextrose | Compatible | pH-dependent degradation |
| Citrate buffers | Reactive | Accelerates deamination |
Applications De Recherche Scientifique
Pharmacokinetics
Cytarabine is poorly absorbed when taken orally due to extensive first-pass metabolism; therefore, it is typically administered via intravenous or subcutaneous routes. Its pharmacokinetic properties include:
- Bioavailability: High when administered intravenously.
- Volume of Distribution: High due to low plasma protein binding.
- Metabolism: Primarily occurs in the liver with renal excretion of metabolites .
Clinical Applications
This compound has a wide range of applications in oncology, particularly for treating different types of leukemia. Below are some key indications:
Liposomal Formulations
Recent advancements have led to the development of liposomal formulations of this compound, which improve its pharmacokinetic profile and reduce systemic toxicity. Liposomal this compound has shown efficacy in treating CNS relapses in ALL and has been compared favorably to traditional therapies .
Efficacy Studies
A study evaluating liposomal this compound for CNS relapse in ALL demonstrated comparable safety and efficacy profiles to methotrexate and traditional this compound formulations. The European Working Group for Adult ALL initiated trials to further explore this application .
Case Studies
-
Case Study: Liposomal this compound in CNS Relapse
- Patient Profile: Adult patient with recurrent ALL.
- Treatment Regimen: Administered liposomal this compound.
- Outcome: Achieved significant CNS response after one cycle; tolerated well without severe adverse effects.
-
Case Study: Differentiation Induction
- Patient Profile: AML patient receiving low-dose this compound.
- Treatment Regimen: Low-dose this compound combined with differentiation agents.
- Outcome: Induced maturation of leukemic cells without significant toxicity, suggesting potential as a differentiation therapy.
Mécanisme D'action
La cytarabine exerce ses effets en inhibant l’ADN polymérase, une enzyme essentielle à la synthèse de l’ADN. Elle est incorporée à l’ADN pendant la phase S du cycle cellulaire, ce qui entraîne la terminaison de l’élongation de la chaîne d’ADN et, finalement, la mort cellulaire. Ce mécanisme rend la this compound particulièrement efficace contre les cellules cancéreuses qui se divisent rapidement .
Comparaison Avec Des Composés Similaires
Composés similaires
Fludarabine : Un autre analogue nucléosidique utilisé dans le traitement de la leucémie.
Cladribine : Utilisé pour traiter la leucémie à tricholeucocytes et la sclérose en plaques.
Gémcitabine : Employée dans le traitement de divers cancers, notamment le cancer du pancréas et le cancer du sein.
Unicité de la cytarabine
La this compound est unique en raison de son mécanisme d’action spécifique, ciblant l’ADN polymérase et son incorporation dans l’ADN. Cette spécificité la rend très efficace dans le traitement de certains types de leucémie. De plus, son utilisation dans des formulations liposomales a montré une cardiotoxicité réduite par rapport à d’autres agents chimiothérapeutiques .
Activité Biologique
Cytarabine, also known as cytosine β-D-arabinofuranoside, is a pyrimidine nucleoside analog primarily used in the treatment of acute myeloid leukemia (AML) and other hematological malignancies. Its biological activity is characterized by its ability to inhibit DNA synthesis, thereby exerting cytotoxic effects on rapidly dividing cells. This article explores the mechanisms of action, pharmacokinetics, clinical efficacy, and recent advancements in drug formulations related to this compound.
This compound's primary mechanism involves its conversion into this compound triphosphate (Ara-CTP) within the cell. This active form competes with deoxycytidine triphosphate (dCTP) for incorporation into DNA during replication. The incorporation of Ara-CTP results in the termination of DNA chain elongation, leading to cell cycle arrest at the S-phase and subsequent apoptosis of malignant cells.
- Key Enzymes Involved :
- Deoxycytidine kinase (DCK) activates this compound by phosphorylating it to Ara-CTP.
- Cytidine deaminase (CDA) is responsible for the catabolism of this compound, influencing its bioavailability and efficacy.
Pharmacokinetics
This compound has a short plasma half-life, typically around 1 to 3 hours, necessitating continuous infusion or frequent dosing to maintain therapeutic levels. Its pharmacokinetic profile can be affected by various factors including patient genetics and concurrent medications.
| Parameter | Value |
|---|---|
| Half-life | 1-3 hours |
| Peak plasma levels | ~0.5 hours post-infusion |
| Bioavailability | Variable (due to CDA activity) |
Clinical Efficacy
This compound is a cornerstone in the treatment regimens for AML and is often used in combination with other agents such as anthracyclines. The efficacy varies among patients due to genetic polymorphisms affecting drug metabolism.
Case Studies
- Ewing Sarcoma : A Phase II study demonstrated that intermediate doses of this compound significantly reduced EWS/FLI1 protein levels in Ewing sarcoma cells both in vitro and in vivo, indicating potential utility beyond traditional leukemias .
- Acute Myeloid Leukemia : Clinical trials have shown that high-dose this compound regimens improve remission rates in patients with AML, particularly those with favorable cytogenetics .
Recent Advancements
Recent research has focused on enhancing the delivery and efficacy of this compound through novel drug formulations:
- Cholic Acid-Cytarabine Conjugates : These liver-targeting conjugates have shown improved absorption and specificity for hepatic tissues, potentially reducing systemic toxicity while maintaining antitumor activity .
- Pharmacogenomic Biomarkers : Studies are exploring genetic markers that predict patient response to this compound therapy, aiming to tailor treatments based on individual metabolic profiles .
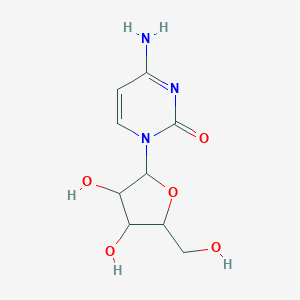
Propriétés
IUPAC Name |
4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16)/t4-,6-,7+,8-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UHDGCWIWMRVCDJ-CCXZUQQUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=CN(C(=O)N=C1N)[C@H]2[C@H]([C@@H]([C@H](O2)CO)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C9H13N3O5 | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
69-74-9 (hydrochloride) | |
| Record name | Cytarabine [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000147944 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID3022877 | |
| Record name | Cytarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022877 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
243.22 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Cytarabine appears as colorless crystals. Used as an antiviral agent., Solid | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>36.5 [ug/mL] (The mean of the results at pH 7.4), Freely soluble, 1 G IN ABOUT 5 ML WATER & 500 ML ALC; 1 G IN ABOUT 1000 ML CHLOROFORM & 300 ML METHANOL, Soluble in water, 4.38e+01 g/L | |
| Record name | SID47193873 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Cytarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00987 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CYTARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3049 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Cytarabine acts through direct DNA damage and incorporation into DNA. Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture. It exhibits cell phase specificity, primarily killing cells undergoing DNA synthesis (S-phase) and under certain conditions blocking the progression of cells from the G1 phase to the S-phase. Although the mechanism of action is not completely understood, it appears that cytarabine acts through the inhibition of DNA polymerase. A limited, but significant, incorporation of cytarabine into both DNA and RNA has also been reported., Cytarabine is converted intracellularly to the nucleotide, cytarabine triphosphate (ara-CTP, cytosine arabinoside triphosphate). Although the exact mechanism(s) of action of cytarabine has not been fully elucidated, cytarabine triphosphate appears to inhibit DNA polymerase by competing with the physiologic substrate, deoxycytidine triphosphate, resulting in the inhibition of DNA synthesis. Although limited, incorporation of cytarabine triphosphate into DNA and RNA may also contribute to the cytotoxic effects of the drug., Cytarabine is a potent immunosuppressant which can suppress humoral and/or cellular immune responses; however, the drug does not decrease preexisting antibody titers and has no effect on established delayed hypersensitivity reactions., Cytarabine liposome injection is a sustained-release formulation of the active ingredient cytarabine designed for direct administration into the cerebrospinal fluid (CSF). Cytarabine is a cell cycle phase-specific antineoplastic agent, affecting cells only during the S-phase of cell division. Intracellularly, cytarabine is converted into cytarabine-5'-triphosphate (ara-CTP), which is the active metabolite. The mechanism of action is not completely understood, but it appears that ara-CTP acts primarily through inhibition of DNA polymerase. Incorporation into DNA and RNA may also contribute to cytarabine cytotoxicity. Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture. /Cytarabine liposome injection/ | |
| Record name | Cytarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00987 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CYTARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3049 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Prisms from 50% ethanol, WHITE TO OFF-WHITE CRYSTALLINE POWDER | |
CAS No. |
147-94-4 | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Cytarabine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=147-94-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cytarabine [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000147944 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cytarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00987 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cytarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3022877 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Cytarabine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.005.188 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CYTARABINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/04079A1RDZ | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CYTARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3049 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
414 to 415 °F (NTP, 1992), 186-188, 212-213 °C, 212 - 213 °C | |
| Record name | CYTARABINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20078 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Cytarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00987 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CYTARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3049 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cytarabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015122 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















