AMD-070
概要
説明
マボリキサフォルは、C-X-C ケモカイン受容体 4 (CXCR4) の経口バイオアベイラビリティのある選択的アンタゴニストです。主に、疣贅、低ガンマグロブリン血症、感染症、およびミエロカテキシス(WHIM)症候群などのまれな一次免疫不全症の治療に開発されています。 マボリキサフォルは、メラノーマを含むさまざまな癌やその他の慢性的な好中球減少症の治療の可能性についても調査されています .
科学的研究の応用
WHIM Syndrome
WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis) is a rare immunodeficiency caused by gain-of-function mutations in the CXCR4 gene. Mavorixafor has been shown to significantly improve clinical outcomes in patients with WHIM syndrome:
- Clinical Trial Results : In a Phase 3 randomized trial, mavorixafor demonstrated a 60% reduction in annualized infection rates compared to placebo (1.7 vs. 4.2 infections) and improved absolute neutrophil counts (ANC) .
- Mechanism : By antagonizing CXCR4, mavorixafor facilitates the release of neutrophils into circulation, thereby enhancing the immune response .
Chronic Neutropenia
Mavorixafor is also being investigated for its efficacy in treating chronic neutropenia (CN), a condition characterized by persistently low levels of neutrophils:
- Phase 2 Trial Findings : A six-month study indicated that mavorixafor treatment led to durable increases in ANC and allowed for significant reductions in granulocyte colony-stimulating factor (G-CSF) dosing without compromising ANC levels .
- Safety Profile : The treatment was well tolerated with mild to moderate side effects predominantly gastrointestinal in nature .
Table 1: Summary of Clinical Trials Involving Mavorixafor
Cancer Treatment
Mavorixafor's role extends into oncology, where it is being explored for its potential to enhance the efficacy of existing cancer therapies:
- Immune Modulation : In a study involving melanoma patients, mavorixafor was administered alongside pembrolizumab to evaluate its effect on immune cell infiltration into tumors. Results suggested improved immune profiles within the tumor microenvironment .
- Potential Indications : Research indicates that mavorixafor could be beneficial for various malignancies including hematologic cancers and solid tumors due to its ability to mobilize immune cells effectively .
作用機序
マボリキサフォルは、CXCR4 受容体に選択的に結合し、その天然リガンドである C-X-C ケモカインリガンド 12(ストローマ由来因子 1 としても知られています)の結合を阻害することで効果を発揮します。この阻害は、CXCR4 シグナル伝達経路の活性化を防ぎ、この経路は免疫細胞のトラフィッキング、造血幹細胞のホーミング、および腫瘍の進行に関与しています。 これらの経路を調節することで、マボリキサフォルは腫瘍微小環境における免疫細胞の浸潤と活性化を強化し、抗腫瘍反応の改善につながります .
類似の化合物との比較
類似の化合物
プレリキサフォル: 非ホジキンリンパ腫および多発性骨髄腫の患者における幹細胞動員に使用される別の CXCR4 アンタゴニストです。
AMD3100: CXCR4 受容体を標的とするバイシクラム分子であり、抗 HIV 特性に関する研究に使用されています.
独自性
マボリキサフォルは、経口バイオアベイラビリティと CXCR4 受容体に対する選択性でユニークです。注射によって投与されるプレリキサフォルや AMD3100 とは異なり、マボリキサフォルは経口で服用できるため、患者にとってより便利です。 さらに、CXCR4 に対する選択性により、標的外効果の可能性が低くなり、安全性のプロファイルが向上します .
生化学分析
Biochemical Properties
AMD-070 interacts with the CXCR4 chemokine receptor, acting as an antagonist . It binds to the transmembrane regions of the coreceptor, blocking the interaction of the CD4-gp120 complex with the ECL2 domain of the CXCR4 coreceptor .
Cellular Effects
This compound has shown to significantly suppress the anchorage-dependent growth, migration, and matrigel invasion of B88-SDF-1 cells . It also inhibits the replication of T-tropic HIV-1 (NL4.3 strain) in MT-4 cells and PBMCs .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to the transmembrane regions of the CXCR4 coreceptor, thereby preventing CXCR4-mediated viral entry of T-cell tropic synctium-inducing HIV . This action blocks the interaction of the CD4-gp120 complex with the ECL2 domain of the CXCR4 coreceptor .
Dosage Effects in Animal Models
It has been shown that this compound (2 mg/kg, p.o.) significantly reduces the number of metastatic lung nodules in mice .
Metabolic Pathways
This compound is primarily metabolized by cytochrome P450 (CYP) 3A4 and to a lesser extent, CYP2D6 . It is also a substrate of P-glycoprotein (P-gp) .
Transport and Distribution
It is known that this compound is a substrate of P-glycoprotein (P-gp), which plays a role in drug transport .
準備方法
合成経路と反応条件
マボリキサフォルの合成には、市販の出発物質から始まる複数のステップが含まれます反応条件には通常、有機溶媒、触媒、および制御された温度の使用が含まれ、高収率と純度が保証されます .
工業生産方法
マボリキサフォルの工業生産は、同様の合成経路に従いますが、大規模製造向けに最適化されています。 これには、連続フロー反応器、自動化された精製システム、および厳格な品質管理対策の使用が含まれ、規制基準との整合性とコンプライアンスが保証されます .
化学反応の分析
反応の種類
マボリキサフォルは、次のものを含むさまざまな化学反応を起こします。
酸化: マボリキサフォルは、特定の条件下で酸化されて酸化誘導体になります。
還元: 還元反応は、キノリン部分の改変に使用できます。
一般的な試薬と条件
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムが含まれます。
還元: 水素化ホウ素ナトリウムや水素化アルミニウムリチウムなどの還元剤が使用されます。
主要な製品
これらの反応から生成される主な生成物には、官能基が改変されたマボリキサフォルのさまざまな誘導体が含まれ、その薬理学的特性についてさらに調査できます .
科学研究アプリケーション
マボリキサフォルは、幅広い科学研究アプリケーションを持っています。
類似化合物との比較
Similar Compounds
Plerixafor: Another CXCR4 antagonist used for stem cell mobilization in patients with non-Hodgkin lymphoma and multiple myeloma.
Uniqueness
Mavorixafor is unique in its oral bioavailability and selectivity for the CXCR4 receptor. Unlike plerixafor and AMD3100, which are administered via injection, mavorixafor can be taken orally, making it more convenient for patients. Additionally, its selectivity for CXCR4 reduces the likelihood of off-target effects, enhancing its safety profile .
生物活性
Mavorixafor is an orally bioavailable, selective antagonist of the CXCR4 chemokine receptor, primarily developed for treating conditions such as WHIM syndrome and chronic neutropenia. Its biological activity is characterized by the modulation of immune cell trafficking, enhancing the mobilization of neutrophils and lymphocytes from the bone marrow into circulation. This article synthesizes findings from various studies to elucidate the biological activity of mavorixafor, focusing on its mechanisms, clinical efficacy, and safety profile.
Mavorixafor functions by inhibiting the CXCR4 receptor, which plays a critical role in the retention of immune cells in the bone marrow. By blocking this receptor, mavorixafor facilitates the release of white blood cells into the bloodstream, thereby increasing their availability for immune responses.
Key Mechanisms:
- Inhibition of CXCR4 : Reduces retention of immune cells in bone marrow.
- Increased Immune Cell Mobilization : Enhances circulating neutrophil and lymphocyte counts.
- Modulation of Tumor Microenvironment (TME) : Increases CD8+ T-cell infiltration in tumors, potentially improving responses to immunotherapies.
Phase Ib Study in Melanoma
A biomarker-driven Phase Ib study (NCT02823405) evaluated mavorixafor's effects on immune cell profiles within the TME of melanoma patients.
| Parameter | Results |
|---|---|
| CD8+ T-cell Infiltration | Increased significantly after 3 weeks of monotherapy |
| Granzyme B Signal | Enhanced expression observed |
| Cytokines (CXCL9, CXCL10) | Elevated levels when combined with pembrolizumab |
| Adverse Events | Diarrhea, fatigue, rash (all ≤ grade 3) |
The study concluded that mavorixafor enhances immune cell infiltration and activation within tumors, suggesting its potential to improve outcomes for patients unresponsive to checkpoint inhibitors .
Phase II Study in WHIM Syndrome
A Phase II open-label study assessed mavorixafor's safety and efficacy in patients with WHIM syndrome.
| Parameter | Results |
|---|---|
| Neutrophil Count (ANC) | Statistically significant increases; maintained >500 cells/µL for 12.6 hours at doses ≥300 mg/day |
| Lymphocyte Count (ALC) | Maintained >1000 cells/µL for up to 16.9 hours |
| Safety Profile | Well-tolerated; no serious adverse events reported |
The study demonstrated that mavorixafor effectively mobilizes neutrophils and lymphocytes, contributing to improved clinical outcomes in patients with this rare immunodeficiency .
Phase III Trial Outcomes
In a Phase III trial involving patients with WHIM syndrome, mavorixafor was compared to placebo over 52 weeks.
| Parameter | Mavorixafor Group | Placebo Group |
|---|---|---|
| Neutrophil Count Increase | Significant increase observed | Not applicable |
| Infection Rate Reduction | Statistically significant decrease | Not applicable |
Adverse effects included low platelet counts and mild rashes but were generally manageable .
Case Studies
- Case Study 1 : A patient with WHIM syndrome treated with mavorixafor exhibited a two-fold increase in ANC after three months, leading to a marked reduction in infection frequency.
- Case Study 2 : In melanoma patients, combination therapy with mavorixafor and pembrolizumab resulted in enhanced tumor-infiltrating lymphocyte activity, correlating with improved overall survival metrics.
特性
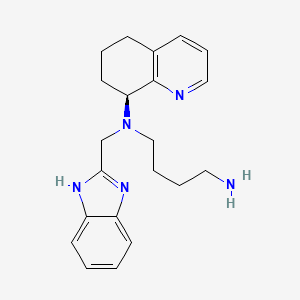
IUPAC Name |
N'-(1H-benzimidazol-2-ylmethyl)-N'-[(8S)-5,6,7,8-tetrahydroquinolin-8-yl]butane-1,4-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H27N5/c22-12-3-4-14-26(15-20-24-17-9-1-2-10-18(17)25-20)19-11-5-7-16-8-6-13-23-21(16)19/h1-2,6,8-10,13,19H,3-5,7,11-12,14-15,22H2,(H,24,25)/t19-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WVLHHLRVNDMIAR-IBGZPJMESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC(C2=C(C1)C=CC=N2)N(CCCCN)CC3=NC4=CC=CC=C4N3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1C[C@@H](C2=C(C1)C=CC=N2)N(CCCCN)CC3=NC4=CC=CC=C4N3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H27N5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60971247 | |
| Record name | N~1~-[(1H-Benzimidazol-2-yl)methyl]-N~1~-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60971247 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
349.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Chemokine receptors expressed on the surface of immune cells are known to play a critical role in virus infection and transmission. CXCR4, and another chemokine receptor CCR5, are involved in HIV infection. The process of HIV entry begins with binding of the viral envelope glycoprotein to both the CD4 receptor and one of only two chemokine receptors, and ends with fusion of viral and cell membranes. Viral entry provides novel therapeutic targets against HIV. To date, at least 3 sub classes of HIV viral entry/fusion inhibitors have emerged: 1. CD4 binding or attachment - targets initial recognition and binding of the viral glycoprotein gp120 to the cell-surface CD4 antigen. 2. Chemokine co-receptor binding - targets binding of virus to the CCR5 or CXCR4 co-receptor. 3. Fusion Inhibition - targets the viral glycoprotein gp41 inhibiting the fusion of virus with the cell. Different strains of HIV prefer one receptor or the other, or may use either receptor to infect cells. * 35% of strains use both CXCR4 and CCR5 * 5% of strains are pure CXCR4 using * 60% of strains are pure CCR5 using * An infected individual may harbor different levels of both CXCR4 and CCR5 using virus * CXCR4 using virus independently predicts CD4 decline and HIV clinical progression and is associated with earlier mortality | |
| Record name | AMD-070 | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB05501 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
558447-26-0 | |
| Record name | Mavorixafor [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0558447260 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | AMD-070 | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB05501 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | N~1~-[(1H-Benzimidazol-2-yl)methyl]-N~1~-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60971247 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | MAVORIXAFOR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0G9LGB5O2W | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details






Synthesis routes and methods II
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。













