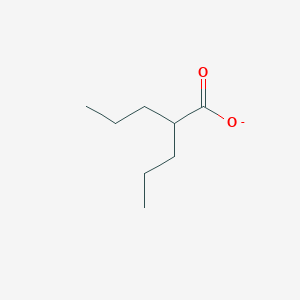
2-propylpentanoate
概要
説明
It is primarily used as a medication to treat epilepsy, bipolar disorder, and to prevent migraine headaches . 2-propylpentanoate was first synthesized in 1882 by Burton, but its anticonvulsant properties were not discovered until 1962 by Pierre Eymard . It is available in various forms, including valproic acid, sodium valproate, and valproate semisodium .
準備方法
Synthetic Routes and Reaction Conditions
2-propylpentanoate can be synthesized through several methods. One common method involves the reaction of valeric acid with sodium hydroxide to produce sodium valproate. Another method involves the esterification of valeric acid with ethanol, followed by hydrolysis to yield valproic acid .
Industrial Production Methods
In industrial settings, valproate is often produced through the oxidation of 2-propylpentanol. This process involves the use of oxidizing agents such as potassium permanganate or chromium trioxide under controlled conditions to yield valproic acid .
化学反応の分析
Types of Reactions
2-propylpentanoate undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized to produce valeric acid.
Reduction: It can be reduced to produce 2-propylpentanol.
Substitution: This compound can undergo substitution reactions to form various derivatives.
Common Reagents and Conditions
Oxidation: Potassium permanganate or chromium trioxide in acidic conditions.
Reduction: Lithium aluminum hydride in anhydrous ether.
Substitution: Halogenating agents such as thionyl chloride or phosphorus tribromide.
Major Products Formed
Oxidation: Valeric acid.
Reduction: 2-propylpentanol.
Substitution: Halogenated derivatives of valproate.
科学的研究の応用
Therapeutic Applications
1. Epilepsy Treatment
- Valproic acid is primarily used as an anticonvulsant medication for managing different types of epilepsy, including generalized tonic-clonic seizures, absence seizures, and complex partial seizures. It is often prescribed as a first-line treatment or as an adjunctive therapy for patients who do not respond adequately to other medications .
2. Bipolar Disorder Management
- The compound is also effective in stabilizing mood in patients with bipolar disorder. It helps prevent manic episodes and is sometimes used in combination with other mood stabilizers .
3. Migraine Prophylaxis
- Valproic acid has been shown to reduce the frequency of migraine attacks and is prescribed for migraine prophylaxis in certain patient populations .
4. Neuropathic Pain
- Research indicates potential analgesic properties of valproic acid, making it a candidate for treating neuropathic pain conditions .
Case Study 1: Efficacy in Epilepsy
A clinical trial conducted in the 1960s demonstrated that valproic acid significantly reduced seizure frequency in patients with refractory epilepsy. The study involved 12 individuals who experienced notable improvements without severe adverse effects . This led to its FDA approval in 1978 for treating various seizure types.
Case Study 2: Valproic Acid Toxicity
A rare case reported in 2023 highlighted the management of valproic acid toxicity requiring hemodialysis. A 47-year-old male presented with severe overdose symptoms after ingesting a high dose of valproic acid. The treatment included hemodialysis, resulting in a marked reduction of serum valproic acid levels and subsequent recovery . This case underscores the importance of monitoring therapeutic levels, especially in patients at risk for toxicity.
Comparative Data Table
| Application | Description | Evidence/Case Studies |
|---|---|---|
| Epilepsy Treatment | Effective for various seizure types | FDA approved since 1978; numerous clinical trials |
| Bipolar Disorder | Stabilizes mood and prevents manic episodes | Supported by multiple studies on mood stabilization |
| Migraine Prevention | Reduces frequency of migraine attacks | Clinical evidence supporting prophylactic use |
| Neuropathic Pain | Potential analgesic properties | Emerging research indicating efficacy |
作用機序
2-propylpentanoate exerts its effects through multiple mechanisms:
GABAergic System: Increases the levels of gamma-aminobutyric acid (GABA) in the brain, enhancing inhibitory neurotransmission.
Sodium Channels: Blocks voltage-gated sodium channels, reducing neuronal excitability.
Histone Deacetylase Inhibition: Inhibits histone deacetylases, leading to changes in gene expression and chromatin structure.
Other Pathways: Affects the Wnt/β-catenin and ERK signaling pathways, and interferes with inositol and arachidonate metabolism.
類似化合物との比較
2-propylpentanoate is unique compared to other anticonvulsants due to its broad spectrum of activity and multiple mechanisms of action. Similar compounds include:
Sodium Butyrate: Another histone deacetylase inhibitor with similar effects on gene expression.
Trichostatin A: A potent histone deacetylase inhibitor used in research.
Divalproex Sodium: A compound of sodium valproate and valproic acid that dissociates to valproate in the gastrointestinal tract.
This compound’s ability to modulate multiple pathways and its broad therapeutic applications make it a valuable compound in both clinical and research settings.
特性
分子式 |
C8H15O2- |
|---|---|
分子量 |
143.2 g/mol |
IUPAC名 |
2-propylpentanoate |
InChI |
InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)/p-1 |
InChIキー |
NIJJYAXOARWZEE-UHFFFAOYSA-M |
SMILES |
CCCC(CCC)C(=O)[O-] |
正規SMILES |
CCCC(CCC)C(=O)[O-] |
同義語 |
2 Propylpentanoic Acid 2-Propylpentanoic Acid Calcium Valproate Convulsofin Depakene Depakine Depakote Dipropyl Acetate Divalproex Divalproex Sodium Ergenyl Magnesium Valproate Propylisopropylacetic Acid Semisodium Valproate Sodium Valproate Valproate Valproate Calcium Valproate Sodium Valproic Acid Valproic Acid, Sodium Salt (2:1) Vupral |
製品の起源 |
United States |
Synthesis routes and methods I
Procedure details











Synthesis routes and methods II
Procedure details














Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。













