ダクラタスビル
概要
説明
ダクラタスビルは、C型肝炎ウイルス(HCV)感染症の治療に他の薬剤と組み合わせて使用される抗ウイルス薬です。ダクリンザという商品名で販売されています。ダクラタスビルは、ウイルス複製に不可欠なHCVタンパク質NS5Aを阻害することにより作用します 。この化合物は、特にHCV遺伝子型1、3、および4に効果的です .
2. 製法
合成経路と反応条件: ダクラタスビルの合成は、市販の出発物質から始めて、複数の工程で実施されます。反応条件は、通常、高収率と高純度を確保するために、有機溶媒、触媒、および制御された温度の使用を含みます。
工業生産方法: ダクラタスビルの工業生産は、同様の合成経路に従いますが、大規模生産に合わせて最適化されています。これには、高効率反応器、連続フロープロセス、および規制基準への準拠と一貫性を確保するための厳格な品質管理対策の使用が含まれます .
作用機序
ダクラタスビルは、HCV非構造タンパク質5A(NS5A)のドメイン1内のN末端に結合することにより、抗ウイルス効果を発揮します。この結合は、ウイルスRNA複製とビリオン集合を阻害し、ウイルスが増殖および拡散することを効果的に阻止します 。NS5Aの阻害は、ウイルス複製複合体の形成を阻害し、ウイルス量の減少と最終的に感染のクリアランスにつながります .
類似の化合物:
ソフォスブビル: HCV治療にダクラタスビルと組み合わせて使用される別の抗ウイルス薬です。HCV RNAポリメラーゼNS5Bを阻害します。
レディパスビル: ダクラタスビルと同様に、NS5Aタンパク質を阻害しますが、薬物動態が異なります。
ベルパタスビル: さまざまなHCV遺伝子型に対してより幅広い活性を示す別のNS5A阻害剤.
ダクラタスビルの独自性: ダクラタスビルは、HCV NS5Aに対する高い選択性と効力によって独特です。薬物動態が良く、1日1回経口投与が可能です。さらに、ダクラタスビルは、進行した肝疾患の患者やHIVに併発感染した患者にも有効性が示されています .
科学的研究の応用
Efficacy in Chronic Hepatitis C Treatment
Clinical Trials and Real-World Studies
- Combination Therapy : Daclatasvir is most commonly used in combination with sofosbuvir and ribavirin. In clinical trials such as the ALLY-1 study, daclatasvir combined with sofosbuvir and ribavirin demonstrated high SVR rates:
- Real-World Effectiveness : Observational studies have shown that daclatasvir plus sofosbuvir achieves cure rates of 90% or higher across various genotypes:
Applications Beyond Hepatitis C
Potential Use in COVID-19 Treatment
Recent studies have explored the use of daclatasvir in treating COVID-19. A meta-analysis indicated that patients treated with daclatasvir and sofosbuvir had significantly lower mortality rates compared to those receiving standard care (5% vs. 20%) and shorter hospital discharge times . This suggests a potential role for daclatasvir as part of therapeutic regimens for COVID-19, particularly in hospitalized patients.
Case Studies
Diverse Patient Populations
- HIV/HCV Coinfection : Daclatasvir has shown promise in treating patients with both HIV and HCV, achieving high SVR rates even among those with advanced liver disease .
- Post-Liver Transplant Recipients : In transplant populations, daclatasvir combined with sofosbuvir resulted in SVR rates exceeding 90%, demonstrating its safety and efficacy in this vulnerable group .
Summary of Key Findings
| Application Area | Study/Trial | SVR Rates (%) | Notes |
|---|---|---|---|
| Chronic HCV Treatment | ALLY-1 Study | Genotype 1: 82% Genotype 3: 95% | Effective for advanced cirrhosis and post-transplant |
| Real-World Effectiveness | Veterans Affairs Cohort | Genotype 2: 94.5% Genotype 3: ~90% | High success rates in routine practice |
| COVID-19 Treatment | Meta-analysis | Mortality: 5% vs. 20% | Significant reduction in death rates |
| HIV/HCV Coinfection | Various Clinical Trials | High SVR Rates | Effective even with advanced liver disease |
生化学分析
Biochemical Properties
Daclatasvir exerts its antiviral action by preventing RNA replication and virion assembly via binding to NS5A, a nonstructural phosphoprotein encoded by HCV . Binding to the N-terminus of the D1 domain of NS5A prevents its interaction with host cell proteins and membranes required for virion replication complex assembly .
Cellular Effects
Daclatasvir has a profound effect on viral load with onset that is more rapid than had been seen previously with either NS3 protease or NS5B polymerase inhibitors . It disrupts the function of new HCV replication complexes by modulating the NS5A phosphorylation status .
Molecular Mechanism
Daclatasvir works by inhibiting the HCV protein NS5A . It targets both the cis- and trans-acting functions of NS5A and disrupts the function of new HCV replication complexes by modulating the NS5A phosphorylation status .
Temporal Effects in Laboratory Settings
Daclatasvir undergoes rapid absorption, with a time to reach maximum plasma concentration of 1–2 h and an elimination half-life of 10 to 14 h observed in single-ascending dose studies . Steady state was achieved by day 4 in multiple-ascending dose studies .
Dosage Effects in Animal Models
In-vivo animal studies suggested that Daclatasvir concentrates in livers (mice, rats, dogs, and monkeys), with liver-to-serum or liver-to-plasma area under the concentration–time curve (AUC) ratios ranging from 1.9 to 17 in the different animal species tested .
Metabolic Pathways
Daclatasvir has low-to-moderate clearance with the predominant route of elimination via cytochrome P450 3A4-mediated metabolism and P-glycoprotein excretion and intestinal secretion . Renal clearance is a minor route of elimination for daclatasvir .
Transport and Distribution
Daclatasvir is transported and distributed within cells and tissues via cytochrome P450 3A4-mediated metabolism and P-glycoprotein excretion and intestinal secretion .
Subcellular Localization
Given its mechanism of action, it is likely to be found in close proximity to the HCV NS5A protein, which is associated with the endoplasmic reticulum membrane where the HCV replication complex is located .
準備方法
Synthetic Routes and Reaction Conditions: The synthesis of daclatasvir involves multiple steps, starting from commercially available starting materials. . The reaction conditions typically involve the use of organic solvents, catalysts, and controlled temperatures to ensure high yield and purity.
Industrial Production Methods: Industrial production of daclatasvir follows a similar synthetic route but is optimized for large-scale production. This involves the use of high-efficiency reactors, continuous flow processes, and stringent quality control measures to ensure consistency and compliance with regulatory standards .
化学反応の分析
反応の種類: ダクラタスビルは、次のようなさまざまな化学反応を受けます。
酸化: ダクラタスビルは特定の条件下で酸化され、酸化誘導体の形成につながることがあります。
還元: 還元反応は、分子内のイミダゾール環または他の官能基を修飾できます。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムが含まれます。
還元: 水素化ホウ素ナトリウムや水素化リチウムアルミニウムなどの還元剤が使用されます。
形成される主要な生成物: これらの反応から形成される主要な生成物には、酸化誘導体、還元アナログ、および薬理学的特性が修飾された置換化合物が含まれます .
類似化合物との比較
Sofosbuvir: Another antiviral medication used in combination with daclatasvir for treating HCV. It inhibits the HCV RNA polymerase NS5B.
Ledipasvir: Similar to daclatasvir, it inhibits the NS5A protein but has different pharmacokinetic properties.
Velpatasvir: Another NS5A inhibitor with a broader spectrum of activity against various HCV genotypes.
Uniqueness of Daclatasvir: Daclatasvir is unique due to its high selectivity and potency against HCV NS5A. It has a well-characterized pharmacokinetic profile, allowing for once-daily oral administration. Additionally, daclatasvir has shown efficacy in patients with advanced liver disease and those co-infected with HIV .
生物活性
Daclatasvir (trade name Daklinza) is a direct-acting antiviral agent primarily used in the treatment of hepatitis C virus (HCV) infections. It acts as an inhibitor of the NS5A protein, which is crucial for viral replication and assembly. This article discusses the biological activity of daclatasvir, including its mechanism of action, efficacy in clinical settings, and emerging research related to its antiviral properties against other viruses such as SARS-CoV-2.
Daclatasvir functions by binding to the N-terminus of the NS5A protein, inhibiting both viral RNA replication and the assembly of new virions. This mechanism is pivotal in reducing the viral load in infected patients and contributes to the overall effectiveness of HCV treatment regimens.
Clinical Trials and Studies
Daclatasvir has demonstrated potent pangenotypic activity against various HCV genotypes (1-4). In clinical trials, it has been shown to significantly improve sustained virological response (SVR) rates when used in combination with other antiviral agents such as sofosbuvir and ribavirin. Key findings from notable studies include:
- COMMAND Trials : These phase 2 trials indicated high SVR rates among patients treated with daclatasvir combined with peginterferon-alpha and ribavirin .
- ALLY Studies : In all-oral combinations, daclatasvir plus sofosbuvir yielded superior response rates, particularly in patients with advanced liver disease and those co-infected with HIV .
Table 1: Summary of Clinical Trial Results for Daclatasvir
| Study Name | Treatment Regimen | SVR Rate (%) | Patient Population |
|---|---|---|---|
| COMMAND | Daclatasvir + PegIFNα + RBV | 90-100 | Genotype 1-4 chronic HCV |
| ALLY | Daclatasvir + Sofosbuvir | 95 | Advanced liver disease, HIV co-infection |
| REAL-WORLD | Daclatasvir + Sofosbuvir + RBV | 92 | Various genotypes |
Antiviral Activity Against SARS-CoV-2
Recent studies have explored the potential of daclatasvir in treating COVID-19. Research indicates that daclatasvir exhibits antiviral activity against SARS-CoV-2, albeit at higher concentrations compared to its efficacy against HCV.
In Vitro Studies
In laboratory settings, daclatasvir showed inhibition of SARS-CoV-2 replication in various cell lines:
- Potency : Daclatasvir inhibited viral replication with effective concentrations (EC50) ranging from 0.6 to 1.1 μM in Vero, HuH-7, and Calu-3 cells .
- Mechanism : It was found to favorably alter the folding of secondary RNA structures in the SARS-CoV-2 genome, inhibiting polymerase reactions essential for viral replication .
Table 2: Comparative Potency of Daclatasvir Against SARS-CoV-2
| Cell Line | EC50 (μM) | Mechanism of Action |
|---|---|---|
| Vero | 0.8 | Inhibition of viral replication |
| HuH-7 | 0.6 | Targeting RNA structure folding |
| Calu-3 | 1.1 | Preventing polymerase activity |
Case Studies
Several case studies have documented the use of daclatasvir in patients with chronic HCV and those affected by COVID-19:
- HCV Treatment : A cohort study involving patients with chronic HCV genotype 3 showed a marked improvement in liver function tests and viral load reduction after treatment with daclatasvir combined with sofosbuvir.
- COVID-19 Treatment : In a small case series, patients treated with a combination of daclatasvir and sofosbuvir exhibited reduced symptom severity and shorter recovery times compared to standard care .
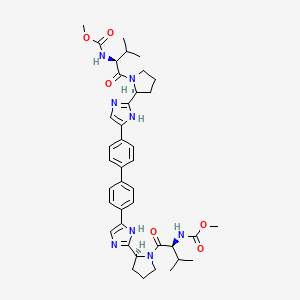
特性
IUPAC Name |
methyl N-[(2S)-1-[(2S)-2-[5-[4-[4-[2-[(2S)-1-[(2S)-2-(methoxycarbonylamino)-3-methylbutanoyl]pyrrolidin-2-yl]-1H-imidazol-5-yl]phenyl]phenyl]-1H-imidazol-2-yl]pyrrolidin-1-yl]-3-methyl-1-oxobutan-2-yl]carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C40H50N8O6/c1-23(2)33(45-39(51)53-5)37(49)47-19-7-9-31(47)35-41-21-29(43-35)27-15-11-25(12-16-27)26-13-17-28(18-14-26)30-22-42-36(44-30)32-10-8-20-48(32)38(50)34(24(3)4)46-40(52)54-6/h11-18,21-24,31-34H,7-10,19-20H2,1-6H3,(H,41,43)(H,42,44)(H,45,51)(H,46,52)/t31-,32-,33-,34-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FKRSSPOQAMALKA-CUPIEXAXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(C(=O)N1CCCC1C2=NC=C(N2)C3=CC=C(C=C3)C4=CC=C(C=C4)C5=CN=C(N5)C6CCCN6C(=O)C(C(C)C)NC(=O)OC)NC(=O)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)[C@@H](C(=O)N1CCC[C@H]1C2=NC=C(N2)C3=CC=C(C=C3)C4=CC=C(C=C4)C5=CN=C(N5)[C@@H]6CCCN6C(=O)[C@H](C(C)C)NC(=O)OC)NC(=O)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C40H50N8O6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID901026404 | |
| Record name | Daclatasvir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID901026404 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
738.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Freely soluble (>700 mg/mL) | |
| Record name | Daclatasvir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09102 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
NS5A is a viral nonstructural phospoprotein that is part of a functional replication complex in charge of viral RNA genome amplification on endoplasmic reticulum membranes. It has the ability to bind to HCV RNA. It is shown to have two distinct functions in HCV RNA replication based on phosphorylated states. Maintaining the HCV replication complex is mediated by the cis-acting function of basally phosphorylated NS5A and the trans-acting function of hyperphosphorylated NS5A modulates HCV assembly and infectious particle formation. Daclatasvir is shown to disrupt hyperphosphorylated NS5A proteins thus interfere with the function of new HCV replication complexes. It is also reported that daclatasvir also blocks both intracellular viral RNA synthesis and virion assembly/secretion in vivo. | |
| Record name | Daclatasvir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09102 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1009119-64-5 | |
| Record name | Daclatasvir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1009119-64-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Daclatasvir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09102 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Daclatasvir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID901026404 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Daclatasvir | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DACLATASVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/LI2427F9CI | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















