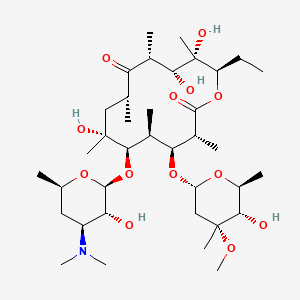
エリスロマイシン
概要
説明
エリスロマイシンは、1952年に初めて発見されたマクロライド系抗生物質ですエリスロマイシンは、呼吸器感染症、皮膚感染症、クラミジア感染症、骨盤内炎症性疾患、梅毒など、さまざまな細菌感染症の治療に広く用いられています . エリスロマイシンは、細菌のタンパク質合成を阻害することで、細菌の増殖を阻止します .
2. 製法
合成経路と反応条件: エリスロマイシンは、通常、細菌であるSaccharopolyspora erythraeaを用いた発酵法によって合成されます。発酵法は、細菌を栄養豊富な培地で培養することで、エリスロマイシンが生成されるプロセスです。 生成されたエリスロマイシンは、その後、さまざまな化学プロセスによって抽出および精製されます .
工業生産方法: 工業的な環境では、エリスロマイシンは発酵槽を用いて大量生産されます。発酵プロセスは、エリスロマイシンの収率を最適化するために、慎重に制御されます。 発酵後、エリスロマイシンは溶媒を用いて抽出され、結晶化およびろ過技術によって精製されます .
3. 化学反応解析
反応の種類: エリスロマイシンは、酸化反応、還元反応、置換反応など、いくつかの化学反応を起こします。 これらの反応は、エリスロマイシンの構造を修飾し、薬理作用が向上した誘導体を生成するために不可欠です .
一般的な試薬と条件:
酸化: エリスロマイシンは、過酸化水素や過マンガン酸カリウムなどの試薬を用いて、制御された条件下で酸化できます。
還元: 還元反応は、水素化ホウ素ナトリウムや水素化リチウムアルミニウムなどの試薬を用いて行うことができます。
置換: 置換反応では、ハロゲンなどの求電子試薬を用いて、エリスロマイシン分子の特定の官能基を置換することがよくあります.
生成される主な生成物: これらの反応によって生成される主な生成物は、アジスロマイシン、クラリスロマイシン、ロキシスロマイシンなど、さまざまなエリスロマイシン誘導体です。 これらの誘導体は、酸性条件下での安定性の悪さなど、エリスロマイシンの欠点を克服するために開発されました .
作用機序
エリスロマイシンは、細菌の50Sリボソームサブユニットに結合し、タンパク質合成を阻害することで作用を発揮します。この作用は、ペプチド鎖の伸長を阻止し、細菌の増殖を効果的に停止させます。 エリスロマイシンの主要な分子標的は細菌のリボソームであり、その作用機序は、新しく合成されたタンパク質が通過する出口トンネルをブロックすることです .
科学的研究の応用
Clinical Applications
Erythromycin is primarily used to treat a variety of bacterial infections. Its spectrum of activity includes both gram-positive and some gram-negative bacteria. Below is a detailed table summarizing its approved uses:
Long-term Effects on Pulmonary Health
A study published in JAMA examined the long-term effects of low-dose erythromycin on patients with pulmonary conditions. The results indicated that erythromycin significantly reduced the rate of pulmonary exacerbations and sputum production in patients with chronic lung diseases, particularly those infected with Pseudomonas aeruginosa .
Ototoxicity Concerns
Research has also highlighted potential ototoxic effects associated with erythromycin. A prospective study found that high doses (4 g/day) were linked to symptomatic ototoxicity, including tinnitus and hearing loss, suggesting a need for careful monitoring at elevated dosages .
Antimicrobial Resistance Trends
Emerging data indicate a concerning rise in erythromycin resistance among certain bacterial strains. A recent study reported a 76% resistance rate among invasive Group A Streptococcus isolates, underscoring the need for ongoing surveillance . This trend poses challenges for effective treatment options and necessitates alternative therapeutic strategies.
Erythromycin in Pediatric Patients
A notable case study involved the use of erythromycin to treat a severe respiratory infection in a pediatric patient with penicillin allergy. The patient showed significant improvement within 48 hours, demonstrating erythromycin's efficacy as an alternative treatment option .
Erythromycin for Acne Treatment
In dermatology, erythromycin's combination with benzoyl peroxide has been shown to reduce acne lesions significantly compared to monotherapy with either agent alone. This combination therapy is particularly beneficial for patients who are intolerant to other systemic treatments .
生化学分析
Biochemical Properties
Erythromycin plays a crucial role in biochemical reactions by interacting with various enzymes, proteins, and other biomolecules. It primarily targets the bacterial ribosome, specifically binding to the 23S ribosomal RNA within the 50S subunit. This binding inhibits the translocation of peptides, effectively halting protein synthesis. Erythromycin also interacts with cytochrome P450 enzymes in the liver, which are involved in its metabolism .
Cellular Effects
Erythromycin affects various types of cells and cellular processes. In bacterial cells, it inhibits protein synthesis by binding to the ribosome, leading to cell death. In eukaryotic cells, erythromycin can influence cell signaling pathways, gene expression, and cellular metabolism. For example, it has been shown to modulate the expression of genes involved in inflammatory responses and can affect mitochondrial function by inhibiting mitochondrial protein synthesis .
Molecular Mechanism
The molecular mechanism of erythromycin involves its binding to the 23S ribosomal RNA in the 50S subunit of the bacterial ribosome. This binding blocks the exit tunnel through which nascent peptides exit the ribosome, thereby inhibiting peptide chain elongation and protein synthesis. Erythromycin’s interaction with the ribosome is highly specific, and its efficacy is influenced by the presence of resistance genes that can modify the ribosomal binding site .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of erythromycin can change over time. Erythromycin is relatively stable under neutral pH but can degrade in acidic conditions. Long-term exposure to erythromycin in vitro can lead to the development of bacterial resistance, characterized by mutations in the ribosomal RNA or the acquisition of resistance genes. Additionally, erythromycin’s stability and efficacy can be influenced by storage conditions and the presence of other compounds .
Dosage Effects in Animal Models
The effects of erythromycin vary with different dosages in animal models. At therapeutic doses, erythromycin effectively treats bacterial infections without significant adverse effects. At higher doses, it can cause gastrointestinal disturbances, hepatotoxicity, and cardiotoxicity. In animal studies, erythromycin has been shown to have a dose-dependent effect on bacterial clearance and the development of resistance .
Metabolic Pathways
Erythromycin is metabolized primarily in the liver by cytochrome P450 enzymes, particularly CYP3A4. The metabolic pathways involve demethylation and hydrolysis, resulting in various metabolites that are excreted in the bile. Erythromycin can also affect the metabolism of other drugs by inhibiting CYP3A4, leading to potential drug-drug interactions .
Transport and Distribution
Erythromycin is transported and distributed within cells and tissues through passive diffusion and active transport mechanisms. It is highly protein-bound in the plasma and can accumulate in tissues such as the liver, lungs, and spleen. Erythromycin’s distribution is influenced by its lipophilicity, allowing it to penetrate cell membranes and reach intracellular targets .
Subcellular Localization
Erythromycin’s subcellular localization is primarily within the cytoplasm, where it exerts its antibacterial effects by targeting the ribosome. It can also localize to the mitochondria in eukaryotic cells, affecting mitochondrial protein synthesis. The localization of erythromycin is influenced by its chemical structure and the presence of specific transporters and binding proteins .
準備方法
Synthetic Routes and Reaction Conditions: Erythromycin is typically synthesized through a fermentation process involving the bacterium Saccharopolyspora erythraea. The fermentation process involves growing the bacterium in a nutrient-rich medium, which leads to the production of erythromycin. The compound is then extracted and purified through various chemical processes .
Industrial Production Methods: In industrial settings, erythromycin is produced on a large scale using fermentation tanks. The fermentation process is carefully controlled to optimize the yield of erythromycin. After fermentation, the compound is extracted using solvents and purified through crystallization and filtration techniques .
化学反応の分析
Types of Reactions: Erythromycin undergoes several types of chemical reactions, including oxidation, reduction, and substitution reactions. These reactions are essential for modifying the structure of erythromycin to produce derivatives with improved pharmacological properties .
Common Reagents and Conditions:
Oxidation: Erythromycin can be oxidized using reagents such as hydrogen peroxide or potassium permanganate under controlled conditions.
Reduction: Reduction reactions can be carried out using reagents like sodium borohydride or lithium aluminum hydride.
Major Products Formed: The major products formed from these reactions include various erythromycin derivatives, such as azithromycin, clarithromycin, and roxithromycin. These derivatives have been developed to overcome some of the limitations of erythromycin, such as poor stability in acidic conditions .
類似化合物との比較
エリスロマイシンは、アジスロマイシン、クラリスロマイシン、ロキシスロマイシンなどの他の化合物を含む、マクロライド系抗生物質のクラスに属します。 これらの化合物は、類似の作用機序を共有していますが、薬物動態特性と活性のスペクトルが異なります .
類似化合物:
アジスロマイシン: 延長された半減期と改善された組織への浸透が特徴です。
クラリスロマイシン: 特定の細菌株に対する活性が高く、酸安定性が向上していることが知られています。
ロキシスロマイシン: エリスロマイシンと比較して、胃腸への耐容性が向上し、半減期が長くなっています.
エリスロマイシンは、幅広い活性を持ち、他のマクロライド系抗生物質の開発のための前駆体としての役割を果たすことから、貴重な抗生物質として位置付けられています。
生物活性
Erythromycin, a macrolide antibiotic discovered in 1952, is widely recognized for its efficacy against a variety of bacterial infections. Its biological activity primarily revolves around its ability to inhibit protein synthesis in susceptible bacteria, making it a crucial agent in treating infections caused by gram-positive bacteria and some gram-negative bacteria. This article delves into the mechanisms, clinical applications, and research findings surrounding erythromycin's biological activity.
Erythromycin exerts its antibacterial effects by binding to the 50S ribosomal subunit of bacterial ribosomes, specifically to the 23S ribosomal RNA . This binding inhibits the translocation step during protein synthesis, effectively halting bacterial growth without directly killing the bacteria (bacteriostatic action) . The specific interactions can be summarized as follows:
- Target : 50S ribosomal subunit
- Action : Inhibition of protein synthesis
- Effect : Bacteriostatic (prevents growth rather than killing)
Antimicrobial Spectrum
Erythromycin is active against a range of microorganisms:
- Gram-positive bacteria : Staphylococcus aureus, Streptococcus pneumoniae
- Gram-negative bacteria : Neisseria, Bordetella, Campylobacter
- Other pathogens : Treponema, Chlamydia
The antibiotic is particularly effective against respiratory pathogens and has been used in the treatment of conditions such as bronchitis and pneumonia.
Clinical Applications
Erythromycin has been employed in various clinical settings. Notable studies and findings include:
- Acute Bronchitis Treatment :
- Pertussis Prophylaxis :
- Infantile Hypertrophic Pyloric Stenosis (IHPS) :
Resistance Patterns
Resistance to erythromycin has become a growing concern. Mechanisms include:
- Methylation of adenine residues in the 23S rRNA leading to reduced binding affinity.
- Efflux pumps that expel the antibiotic from bacterial cells.
- Enzymatic degradation through hydrolysis .
These resistance mechanisms highlight the need for ongoing surveillance and research into alternative therapies.
Case Study 1: Erythromycin in Tuberculosis Treatment
A study presented at a conference highlighted the use of erythromycin as an adjunctive treatment for drug-sensitive and multi-drug resistant tuberculosis. The results indicated that erythromycin could enhance treatment outcomes when combined with standard anti-tubercular therapies .
Case Study 2: Erythromycin vs. Placebo in Respiratory Infections
In a randomized controlled trial involving 63 adults with acute bronchitis, those treated with erythromycin reported significantly better outcomes regarding cough and sputum production compared to the placebo group (P < .05) .
Summary of Findings
The following table summarizes key findings related to erythromycin's biological activity:
特性
IUPAC Name |
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ULGZDMOVFRHVEP-RWJQBGPGSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC[C@@H]1[C@@]([C@@H]([C@H](C(=O)[C@@H](C[C@@]([C@@H]([C@H]([C@@H]([C@H](C(=O)O1)C)O[C@H]2C[C@@]([C@H]([C@@H](O2)C)O)(C)OC)C)O[C@H]3[C@@H]([C@H](C[C@H](O3)C)N(C)C)O)(C)O)C)C)O)(C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C37H67NO13 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4022991 | |
| Record name | Erythromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4022991 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
733.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Soluble in water at 2mg/ml, WHITE OR SLIGHTLY YELLOW CRYSTALS OR POWDER, PRACTICALLY ODORLESS. SOLN IS ALKALINE TO LITMUS. SOL IN METHANOL, CHLOROFORM. /Erythromycin stearate/, WHITE OR SLIGHTLY YELLOW, CRYSTALLINE POWDER. ODORLESS OR PRACTICALLY SO. PRACTICALLY TASTELESS. PKA 7. FREELY SOL IN ACETONE & CHLOROFORM; SOL IN 95% ETHANOL & BENZENE; SPARINGLY SOL IN ETHER; VERY SLIGHTLY SOL IN WATER. /Erythromycin ethyl succinate/, FREELY SOLUBLE IN ALC, SOL IN POLYETHYLENE GLYCOL /Erythromycin ethyl succinate, Very soluble in acetone, ethyl ether, ethanol, chloroform, Freely soluble in alcohols, acetone, chloroform, acetonitrile, ethyl acetate; moderately soluble in ether, ethylene dichloride, amyl acetate, Solubility in water: approx 2 mg/ML, 4.59e-01 g/L | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
In order to replicate, bacteria require a specific process of protein synthesis, enabled by ribosomal proteins. Erythromycin acts by inhibition of protein synthesis by binding to the 23S ribosomal RNA molecule in the 50S subunit of ribosomes in susceptible bacterial organisms. It stops bacterial protein synthesis by inhibiting the transpeptidation/translocation step of protein synthesis and by inhibiting the assembly of the 50S ribosomal subunit. This results in the control of various bacterial infections. The strong affinity of macrolides, including erythromycin, for bacterial ribosomes, supports their broad‐spectrum antibacterial activities., Macrolide antibiotics are bacteriostatic agents that inhibit protein synthesis by binding reversibly to 50S ribosomal subunits of sensitive microorganisms, at or very near the site that binds chloramphenicol. Erythromycin does not inhibit peptide bond formation per se, but rather inhibits the translocation step wherein a newly synthesized peptidyl tRNA molecule moves from the acceptor site on the ribosome to the peptidyl donor site. Gram-positive bacteria accumulate about 100 times more erythromycin than do gram-negative bacteria. Cells are considerably more permeable to the un-ionized form of the drug, which probably explains the increased antimicrobial activity at alkaline pH., ... /Erythromycin/ inhibits the growth of susceptible organisms (principally Propionibacterium acnes) on the surface of the skin and reduces the concn of free fatty acids in sebum ... The reduction in free fatty acids in sebum may be an indirect result of the inhibition of lipase-producing organisms which convert triglycerides into free fatty acids or may be a direct result of interference with lipase production in these organisms. /In acne treatment regimens/, Although stromal-derived factor-1 (SDF-1) via its cognate receptor CXCR4 is assumed to play a critical role in migration of endothelial cells during new vessel formation after tissue injury, CXCR4 expression on endothelial cells is strictly regulated. Erythromycin (EM), a 14-membered ring macrolide, has an anti-inflammatory effect that may account for its clinical benefit in the treatment of chronic inflammatory diseases. However, the effects of EM on endothelial cells and especially their expression of CXCR4 have not been fully evaluated. In this study, we demonstrated that EM markedly induced CXCR4 surface expression on microvascular endothelial cells in vitro and lung capillary endothelial cells in vivo. This ability to induce CXCR4 surface expression on endothelial cells was restricted to 14-membered ring macrolides and was not observed in other antibiotics including a 16-membered ring macrolide, josamycin. Furthermore, this EM-induced expression of CXCR4 on endothelial cells was functionally significant as demonstrated by chemotaxis assays in vitro. These findings suggest that EM-induced CXCR4 surface expression on endothelial cells may promote migration of CXCR4-expressing endothelial cells into sites of tissue injury, which may be associated with the known anti-inflammatory activity of this macrolide. | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Hydrated crystals from water, Crystals from water, White or slightly yellow crystals or powder | |
CAS No. |
114-07-8, 82343-12-2, 215031-94-0, 7540-22-9 | |
| Record name | Erythromycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=114-07-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Erythromycin [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000114078 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | N-Methylerythromycin A | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0082343122 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Erythromycin C-13 | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0215031940 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | erythromycin | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=756759 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Erythromycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4022991 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Erythromycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.003.673 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Erythromycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Erythromycin | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/63937KV33D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
133-135, 191 °C, After melting /at 135-140 °C, it/ resolidifies with second melting point 190-193 °C. ... Readily forms salts with acids, MP: 92 °C. Slightly soluble in ethanol, ethyl ether, chloroform; insoluble in water. /Erythromycin stearate/, Crystals from acetone aqueous. MP: 222 °C. MW: 862.05. /Erythromycin ethyl succinate/ | |
| Record name | Erythromycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00199 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Erythromycin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3074 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Erythromycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014344 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















