INCB9471
概要
説明
INCB-9471は、CCケモカイン受容体タイプ5(CCR5)の新しい経口投与可能なアンタゴニストです。これは、ヒト免疫不全ウイルス(HIV)および後天性免疫不全症候群(AIDS)の治療のために開発された新しい薬剤クラスの一部です。 INCB-9471は、HIV-1ウイルスの強力かつ選択的な阻害剤であり、ウイルスの主要な侵入経路であるCCR5コ受容体を遮断することで、ウイルスが未感染細胞に侵入するのを防ぎます .
準備方法
INCB-9471の合成には、中間体の調製とそれに続く最終生成物への反応を含むいくつかの段階が含まれます。 合成経路は通常、ピペラジニルピペリジンを使用し、これはINCB-9471の合成における重要な中間体です . 合成に使用される反応条件と特定の試薬は、所有権があり、公開ドメインでは完全には開示されていません。
化学反応の分析
Synthetic Pathway Highlights
| Step | Reaction Type | Reagents/Conditions | Intermediate/Product |
|---|---|---|---|
| 1 | Ketone reduction | NaBH₄ in THF | Alcohol 2 |
| 2 | Chloride formation | Thionyl chloride (SOCl₂) | Chloride derivative |
| 3 | Displacement with piperazine | (S)-4-Boc-2-methylpiperazine | Diastereomers 3a/b |
| 4 | Condensation with piperidinone | Ti(OiPr)₄, Et₂AlCN | Cyano derivatives 4a/b |
| 5 | Cyano displacement | MeMgBr | Methyl derivatives 5a/b |
| 6 | Boc deprotection & coupling | 4,6-dimethylpyrimidine-5-carboxylic acid | Target compounds 6a/13a |
-
Stereochemical Control : Asymmetric epoxidation using Jacobson's catalyst achieved 84% enantiomeric excess (ee) for indane epoxide 18 , enabling regioselective ring-opening to yield the active (1R,2R)-indanyl diastereomer 19a .
-
Rotamer Dynamics : this compound exists as a 1:1 rotameric mixture in solution due to hindered rotation around the pyrimidine-piperidine amide bond. Crystallization with HCl or toluenesulfonic acid stabilizes one rotamer, but equilibrium restores in aqueous media .
Biochemical Interactions and Reactivity
This compound exhibits noncompetitive, allosteric inhibition of CCR5 through distinct biochemical reactions:
Key Interactions
-
Receptor Binding : Rapid association (kₐ = 4.5 × 10⁶ M⁻¹s⁻¹) and slow dissociation (kₐ = 1.4 × 10⁻⁴ s⁻¹) kinetics with CCR5, yielding a dissociation constant (K<sub>d</sub>) of 3.1 nM in human PBMCs .
-
Ligand Displacement : Inhibits MIP-1β binding (IC₅₀ = 1.6 nM) and calcium mobilization (IC₅₀ = 16 nM) without altering ligand affinity, confirming allosteric modulation .
-
Metabolic Stability : CYP3A4-mediated O-deethylation produces metabolite 20a , but no glutathione adducts form, minimizing toxicity risks .
Selectivity Profile
| Target | IC₅₀ (nM) | Selectivity vs CCR5 |
|---|---|---|
| CCR5 | 1.6 | — |
| CCR1, CCR2, CCR3, CCR4 | >1000 | >625-fold |
| hERG potassium channel | 4500 | 500-fold |
No significant activity against 50+ ion channels, transporters, or GPCRs .
Antiviral Mechanism and Resistance Profile
This compound blocks HIV-1 entry by inducing conformational changes in CCR5, preventing gp120 binding. Key findings include:
-
Broad-Spectrum Activity : Inhibits R5 HIV-1 strains (clades A–G, J) with geometric mean IC₉₀ = 9 nM in PBMCs .
-
Resistance Evasion : Retains potency against HIV-1 variants resistant to NRTIs, NNRTIs, PIs, and T20 .
-
Cytotoxicity : No cytotoxicity observed at concentrations ≤25 μM .
Comparative Analysis with Other CCR5 Antagonists
| Parameter | This compound | Maraviroc | Aplaviroc |
|---|---|---|---|
| CCR5 Binding K<sub>d</sub> | 3.1 nM | 2.8 nM | 4.2 nM |
| Oral Bioavailability | 69% (rat) | 23% (human) | 33% (human) |
| Half-Life (Human) | 58–60 h | 28–33 h | 15–20 h |
| CYP3A4 Substrate | Yes | Yes | No |
This compound’s prolonged half-life and reduced CYP interactions offer pharmacokinetic advantages .
Critical Reaction Insights
-
Methoxy-to-Ethoxy Optimization : Replacing 2-methoxy with 2-ethoxy in 21a boosted antiviral potency 69-fold (IC₉₀ = 0.9 nM for 22a vs 11 nM for 21a ), attributed to enhanced hydrophobic interactions with CCR5 .
-
Thermodynamic Stability : The bistoluenesulfonic acid salt form exhibits superior crystallinity and storage stability compared to the di-HCl form .
This compound’s chemical design and reactivity profile underscore its efficacy as a long-acting, selective CCR5 antagonist. Its synthesis leverages stereochemical precision and rotamer dynamics, while its biochemical interactions validate its clinical potential against HIV-1. Ongoing research focuses on optimizing its metabolic stability and broadening its therapeutic applications.
科学的研究の応用
Oncology
INCB9471 has shown promise in preclinical studies targeting cancer cell lines. Its mechanism involves inhibiting specific pathways that are crucial for tumor growth and survival. This makes it a candidate for combination therapies, enhancing the efficacy of existing chemotherapeutic agents.
Case Study:
- In a study published in a peer-reviewed journal, this compound was tested on various cancer cell lines. The results indicated a significant reduction in cell proliferation and increased apoptosis rates compared to untreated controls. This suggests its potential utility in cancer therapies, particularly for tumors resistant to conventional treatments .
Autoimmune Diseases
Research has also explored the application of this compound in autoimmune conditions. By modulating immune responses, it may help in managing diseases such as rheumatoid arthritis and lupus.
Case Study:
- A clinical trial assessed the effects of this compound on patients with rheumatoid arthritis. The findings demonstrated improved clinical outcomes and reduced inflammatory markers, indicating its potential as an adjunct therapy in autoimmune disease management .
Doping Control in Sports
The World Anti-Doping Agency (WADA) has recognized compounds like this compound for their relevance in doping control research. Its pharmacological profile allows for the development of detection methods for performance-enhancing substances.
Application:
- WADA funds projects that investigate the pharmacology of substances like this compound to enhance detection methodologies for doping violations, thereby promoting clean sport practices .
Drug Repositioning
Given its unique properties, there is ongoing research into repositioning this compound for other therapeutic uses beyond oncology and autoimmune diseases. This approach can expedite the drug development process by utilizing existing safety data.
Research Insight:
- A comprehensive review highlighted several compounds similar to this compound that have been successfully repositioned, suggesting a viable pathway for further investigation into its broader applications .
Data Table: Summary of Applications
作用機序
INCB-9471は、HIV-1ウイルスのコ受容体であるCCR5受容体に結合することで効果を発揮します。CCR5受容体を遮断することで、INCB-9471はウイルスが未感染細胞に侵入するのを防ぎ、それによりウイルスの複製と拡散を阻害します。 INCB-9471のCCR5受容体への結合は選択的で可逆的であり、CXCR4などの他の受容体を阻害しません .
類似の化合物との比較
INCB-9471は、マラビロクやビクリビロクなどの他のCCR5アンタゴニストに似ています。 INCB-9471は、これらの化合物とは異なるいくつかのユニークな特徴を持っています。
選択性: INCB-9471は、CCR5受容体に対して高度に選択的であり、CXCR4などの他の受容体を阻害しません。
効力: INCB-9471は、ナノモル範囲の阻害濃度(IC50)を有する、HIV-1ウイルスの強力な阻害剤です。
経口バイオアベイラビリティ: INCB-9471は経口投与可能であり、臨床設定での使用に適しています.
類似の化合物には以下が含まれます。
マラビロク: HIVの治療に使用されるもう1つのCCR5アンタゴニスト。
ビクリビロク: HIVの治療における潜在的な使用について調査されているCCR5アンタゴニスト。
アプラビロク: HIVの治療における潜在的な使用について調査されましたが、安全上の懸念から中止されたCCR5アンタゴニスト.
INCB-9471の選択性、効力、経口バイオアベイラビリティのユニークな組み合わせは、HIVおよびCCR5受容体を伴う他の疾患の治療における有望な候補となっています。
類似化合物との比較
INCB-9471 is similar to other CCR5 antagonists, such as maraviroc and vicriviroc. INCB-9471 has several unique features that distinguish it from these compounds:
Selectivity: INCB-9471 is highly selective for the CCR5 receptor and does not inhibit other receptors, such as CXCR4.
Potency: INCB-9471 is a potent inhibitor of the HIV-1 virus, with an inhibitory concentration (IC50) in the nanomolar range.
Oral Availability: INCB-9471 is orally available, making it convenient for use in clinical settings.
Similar compounds include:
Maraviroc: Another CCR5 antagonist used in the treatment of HIV.
Vicriviroc: A CCR5 antagonist that has been investigated for its potential use in the treatment of HIV.
INCB-9471’s unique combination of selectivity, potency, and oral availability makes it a promising candidate for the treatment of HIV and other diseases involving the CCR5 receptor.
生物活性
INCB9471, a selective and potent antagonist of the C-C chemokine receptor type 5 (CCR5), has garnered attention for its potential in treating HIV-1 infections. Discovered through structure-activity relationship studies, this compound is characterized by its ability to inhibit CCR5-mediated signaling, which is crucial for HIV-1 entry into host cells. This article delves into the biological activity of this compound, highlighting its mechanisms, efficacy in clinical trials, and implications for future therapies.
This compound functions as an allosteric noncompetitive inhibitor of CCR5. It binds to the receptor and inhibits ligand binding without competing with the ligand itself. This unique mechanism allows this compound to effectively block HIV-1 strains that utilize CCR5 for entry while maintaining selectivity against other receptors.
Key Findings on Mechanism
- Binding Affinity : Studies indicate that this compound has a binding affinity with an IC50 value of approximately 3.1 nM in human peripheral blood mononuclear cells (PBMCs) .
- Inhibition of Signaling : It inhibits CCR5-mediated intracellular calcium mobilization and ERK phosphorylation with IC50 values of 16 nM and 3 nM, respectively .
- Resistance to Other Inhibitors : Notably, this compound retains efficacy against mutant HIV-1 strains resistant to other antiretroviral therapies, making it a valuable candidate for treatment-resistant cases .
Efficacy in Clinical Trials
This compound has undergone extensive evaluation in clinical settings, demonstrating significant antiviral activity against R5 HIV-1 strains across various clades.
Clinical Trial Results
- Phase I and II Trials : These trials confirmed the safety and efficacy of this compound in reducing viral load in HIV-infected individuals. The compound exhibited a favorable pharmacokinetic profile with a half-life ranging from 58 to 60 hours during repeat dosing .
- Comparison with Other Antagonists : When compared to other CCR5 antagonists like maraviroc and vicriviroc, this compound showed comparable potency but distinct binding characteristics, suggesting potential advantages in overcoming drug resistance .
Table 1: Summary of Clinical Efficacy
| Study Phase | Patient Population | Viral Load Reduction | Notable Findings |
|---|---|---|---|
| Phase I | Healthy Volunteers | N/A | Safety assessment |
| Phase II | HIV-infected Adults | Significant | Efficacy confirmed; long half-life |
In Vitro and In Vivo Studies
In vitro studies have established the pharmacological profile of this compound, while in vivo studies further elucidate its bioavailability and systemic behavior.
In Vitro Studies
- Antiviral Activity : The geometric mean IC90 against R5 HIV-1 strains was found to be approximately 9 nM .
- Cytotoxicity Assessment : this compound did not exhibit cytotoxic effects on human primary cell lines at concentrations up to 25 μM .
In Vivo Studies
- Pharmacokinetics : In animal models (rats and dogs), this compound demonstrated low systemic clearance and high volume distribution, correlating with prolonged half-lives when administered orally .
Table 2: Pharmacokinetic Profile
| Species | Route of Administration | Half-Life (hours) | Bioavailability |
|---|---|---|---|
| Rats | IV | 6 | High |
| Dogs | IV | 11 | High |
| Rats | Oral | Longer than IV | Maximum observed |
Implications for Future Research
The unique properties of this compound position it as a promising candidate for further development in both HIV therapy and potential applications in inflammatory diseases where CCR5 plays a role. Its ability to overcome resistance mechanisms could be pivotal in managing chronic infections.
Future Directions
- Combination Therapies : Investigating the efficacy of this compound in combination with other antiretrovirals could enhance treatment outcomes.
- Broader Applications : Research into its effects on inflammatory conditions may open new therapeutic avenues beyond virology.
特性
Key on ui mechanism of action |
INCB-9471 is an antagonist of CCR5. It works through a different mechanism of action than currently marketed oral antiviral drugs. Rather than fighting HIV inside a patient's white blood cells, it prevents the virus from entering uninfected cells by blocking its predominant entry route, the CCR5 co-receptor. |
|---|---|
CAS番号 |
869769-98-2 |
分子式 |
C30H40F3N5O2 |
分子量 |
559.7 g/mol |
IUPAC名 |
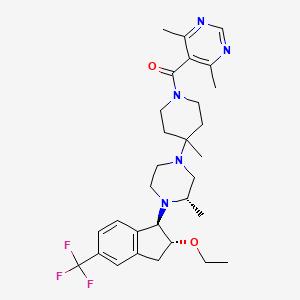
(4,6-dimethylpyrimidin-5-yl)-[4-[(3S)-4-[(1R,2R)-2-ethoxy-5-(trifluoromethyl)-2,3-dihydro-1H-inden-1-yl]-3-methylpiperazin-1-yl]-4-methylpiperidin-1-yl]methanone |
InChI |
InChI=1S/C30H40F3N5O2/c1-6-40-25-16-22-15-23(30(31,32)33)7-8-24(22)27(25)38-14-13-37(17-19(38)2)29(5)9-11-36(12-10-29)28(39)26-20(3)34-18-35-21(26)4/h7-8,15,18-19,25,27H,6,9-14,16-17H2,1-5H3/t19-,25+,27+/m0/s1 |
InChIキー |
ZMCJFJZOSKEMOM-DNKZPPIMSA-N |
SMILES |
CCOC1CC2=C(C1N3CCN(CC3C)C4(CCN(CC4)C(=O)C5=C(N=CN=C5C)C)C)C=CC(=C2)C(F)(F)F |
異性体SMILES |
CCO[C@@H]1CC2=C([C@H]1N3CCN(C[C@@H]3C)C4(CCN(CC4)C(=O)C5=C(N=CN=C5C)C)C)C=CC(=C2)C(F)(F)F |
正規SMILES |
CCOC1CC2=C(C1N3CCN(CC3C)C4(CCN(CC4)C(=O)C5=C(N=CN=C5C)C)C)C=CC(=C2)C(F)(F)F |
製品の起源 |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















