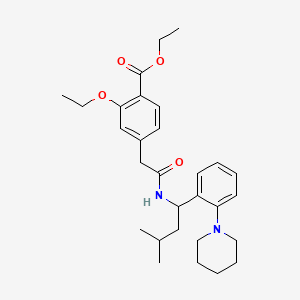
Repaglinide ethyl ester
Übersicht
Beschreibung
Repaglinide ethyl ester is a chemical compound that serves as an intermediate in the synthesis of repaglinide, a medication used to manage type 2 diabetes mellitus. Repaglinide is a non-sulfonylurea insulin secretagogue that helps regulate blood glucose levels by stimulating insulin release from pancreatic beta cells .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions
Repaglinide ethyl ester can be synthesized through various synthetic routes. One common method involves the esterification of 3-hydroxyphenylacetic acid, followed by formylation, oxidation, etherification, and selective hydrolysis . The reaction conditions, such as temperature, time, solvent, and substrate ratios, are optimized to achieve high yields and purity .
Industrial Production Methods
In industrial settings, the production of this compound involves large-scale synthesis using similar reaction steps. The process is designed to be scalable and environmentally friendly, minimizing waste and optimizing resource utilization .
Analyse Chemischer Reaktionen
Types of Reactions
Repaglinide ethyl ester undergoes various chemical reactions, including:
Oxidation: Conversion of functional groups to higher oxidation states.
Reduction: Conversion of functional groups to lower oxidation states.
Substitution: Replacement of one functional group with another.
Common Reagents and Conditions
Common reagents used in these reactions include oxidizing agents like potassium permanganate, reducing agents like lithium aluminum hydride, and substitution reagents like ethyl bromide . The reactions are typically carried out under controlled conditions to ensure high selectivity and yield.
Major Products Formed
The major products formed from these reactions include various intermediates that are further processed to produce repaglinide .
Wissenschaftliche Forschungsanwendungen
Repaglinide ethyl ester has several scientific research applications:
Chemistry: Used as an intermediate in the synthesis of repaglinide and other related compounds.
Medicine: Essential in the development of repaglinide, a drug used to manage type 2 diabetes mellitus.
Industry: Utilized in the large-scale production of repaglinide, ensuring consistent quality and supply.
Wirkmechanismus
Repaglinide ethyl ester itself does not have a direct mechanism of action, as it is an intermediate compound. repaglinide, the final product, exerts its effects by inhibiting ATP-sensitive potassium channels in pancreatic beta cells. This inhibition leads to membrane depolarization, opening of calcium channels, and subsequent insulin release .
Vergleich Mit ähnlichen Verbindungen
Similar Compounds
Similar compounds to repaglinide ethyl ester include:
Nateglinide: Another meglitinide class drug used to manage type 2 diabetes.
Mitiglinide: A similar insulin secretagogue with a different chemical structure.
Uniqueness
This compound is unique due to its specific role as an intermediate in the synthesis of repaglinide. Its optimized synthetic route and reaction conditions make it a valuable compound in the pharmaceutical industry .
Eigenschaften
Molekularformel |
C29H40N2O4 |
|---|---|
Molekulargewicht |
480.6 g/mol |
IUPAC-Name |
ethyl 2-ethoxy-4-[2-[[3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoate |
InChI |
InChI=1S/C29H40N2O4/c1-5-34-27-19-22(14-15-24(27)29(33)35-6-2)20-28(32)30-25(18-21(3)4)23-12-8-9-13-26(23)31-16-10-7-11-17-31/h8-9,12-15,19,21,25H,5-7,10-11,16-18,20H2,1-4H3,(H,30,32) |
InChI-Schlüssel |
FTCMVLQJMIXDSI-UHFFFAOYSA-N |
Kanonische SMILES |
CCOC1=C(C=CC(=C1)CC(=O)NC(CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)OCC |
Herkunft des Produkts |
United States |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details




Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.
![3-(1,3-Benzodioxol-5-yl)-3-[(2-phenylacetyl)amino]propanoic acid](/img/structure/B8791354.png)
![3-(4,4-Dimethyl-2-oxo-2,4-dihydro-1H-benzo[D][1,3]oxazin-6-YL)benzonitrile](/img/structure/B8791411.png)
![4-{[4-(Methanesulfonyl)benzene-1-sulfonyl]amino}benzoic acid](/img/structure/B8791437.png)
![2-Amino-5-methyl-4,5-dihydropyrazolo[1,5-a]pyrazin-6(7H)-one](/img/structure/B8791444.png)
