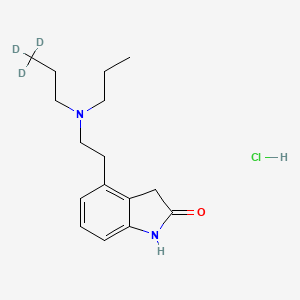
Ropinirole D3 Hydrochloride
Overview
Description
Ropinirole D3 Hydrochloride is a deuterated form of Ropinirole Hydrochloride, a non-ergoline dopamine agonist. It is primarily used in the treatment of Parkinson’s disease and Restless Legs Syndrome. The deuterated version, this compound, contains deuterium atoms, which can enhance the pharmacokinetic properties of the compound, potentially leading to improved efficacy and reduced side effects .
Preparation Methods
Synthetic Routes and Reaction Conditions
The synthesis of Ropinirole D3 Hydrochloride involves the incorporation of deuterium atoms into the Ropinirole molecule This can be achieved through various methods, including the use of deuterated reagents or solvents during the synthesis processThe final step involves the formation of the hydrochloride salt .
Industrial Production Methods
Industrial production of this compound follows similar steps to the laboratory synthesis but on a larger scale. The process involves the use of high-purity deuterated reagents and solvents to ensure the incorporation of deuterium atoms. The reaction conditions are optimized to maximize yield and purity, and the final product is subjected to rigorous quality control measures to ensure its suitability for pharmaceutical use .
Chemical Reactions Analysis
Types of Reactions
Ropinirole D3 Hydrochloride undergoes various chemical reactions, including:
Oxidation: The compound can be oxidized to form various metabolites.
Reduction: Reduction reactions can occur, particularly in the presence of reducing agents.
Substitution: The compound can undergo substitution reactions, particularly at the dipropylaminoethyl side chain.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are commonly used.
Substitution: Substitution reactions often involve the use of halogenating agents or nucleophiles.
Major Products Formed
The major products formed from these reactions include various metabolites and derivatives of this compound, which can have different pharmacological properties .
Scientific Research Applications
Ropinirole hydrochloride is a non-ergoline dopamine agonist primarily used to manage Parkinson's disease and Restless Legs Syndrome (RLS) . It can be used as early therapy without concomitant levodopa and as an adjunct to levodopa . While the precise mechanism of action for Parkinson's disease is not fully understood, ropinirole is thought to selectively stimulate dopamine D2 receptors in the caudate-putamen system, a region of the brain affecting body movement . Similarly, its action in RLS is believed to be related to its dopamine receptor stimulation . Ropinirole has a high affinity for D3 receptors, which are concentrated in the limbic areas of the brain and may be responsible for some of the neuropsychiatric effects .
Clinical Applications and Efficacy
Parkinson's Disease: Ropinirole is effective in treating Parkinson's disease, which is characterized by a marked dopamine deficiency in the nigrostriatal system . Ropinirole alleviates this deficiency by stimulating striatal dopamine receptors . Clinical studies have demonstrated the effectiveness of ropinirole in improving locomotor activity and reducing disability in animal models of Parkinson's disease .
Restless Legs Syndrome: Ropinirole is also used to treat Restless Legs Syndrome . RLS is a difficult to define condition that is possibly more prevalent than previously thought .
Amyotrophic Lateral Sclerosis: Ropinirole hydrochloride is being explored as a potential remedy for amyotrophic lateral sclerosis (ALS) . A clinical trial (ROPALS) is underway to assess the safety, tolerability, and efficacy of ropinirole hydrochloride extended-release tablets in patients with ALS . An in vitro model showed that ropinirole hydrochloride had significantly superior anti-ALS therapeutic activity compared with riluzole and edaravone, which are already-approved drugs for ALS .
Dosage and Administration
For Parkinson's disease, ropinirole tablets can be used as early therapy without levodopa or as an adjunct to levodopa . Oral clearance of Ropinirole Tablets is reduced in patients older than 65 years of age, however, the dosing of ropinirole for elderly patients can be titrated in the normal manner .
Ropinirole is available in both immediate-release and prolonged-release formulations . Immediate-release tablets are typically taken three times daily due to ropinirole's short elimination half-life of approximately 6 hours . A low starting dose of 0.75 mg/day is recommended, with a slow initial titration regimen of 0.75 mg weekly over the first four weeks . Prolonged-release tablets allow for once-daily administration, with a higher starting dose of 2 mg/day and a larger up-titration increment of 2 mg weekly .
Adverse Effects
Mechanism of Action
Ropinirole D3 Hydrochloride exerts its effects by stimulating dopamine D2 and D3 receptors in the brain. This leads to an increase in dopamine levels, which helps to regulate motor control and reduce symptoms of Parkinson’s disease and Restless Legs Syndrome. The deuterium atoms in this compound can enhance the stability and bioavailability of the compound, potentially leading to improved therapeutic outcomes .
Comparison with Similar Compounds
Similar Compounds
Pramipexole Dihydrochloride: Another dopamine agonist used in the treatment of Parkinson’s disease and Restless Legs Syndrome.
Rotigotine: A dopamine agonist available as a transdermal patch for the treatment of Parkinson’s disease.
Apomorphine: A dopamine agonist used for the treatment of advanced Parkinson’s disease.
Uniqueness
Ropinirole D3 Hydrochloride is unique due to the incorporation of deuterium atoms, which can enhance its pharmacokinetic properties. This can lead to improved efficacy, reduced side effects, and potentially longer duration of action compared to non-deuterated dopamine agonists .
Biological Activity
Ropinirole D3 Hydrochloride is a potent non-ergoline dopamine agonist primarily used in the treatment of Parkinson's disease (PD) and restless legs syndrome (RLS). This article delves into its biological activity, mechanisms of action, pharmacokinetics, and relevant clinical findings.
Ropinirole acts predominantly on dopamine D2 and D3 receptors, with a higher affinity for D3 receptors. This selectivity allows it to effectively stimulate postsynaptic dopamine receptors, compensating for the dopamine deficiency characteristic of PD. The mechanism involves the inhibition of adenylyl cyclase and calcium channels while activating potassium channels, leading to enhanced dopaminergic neurotransmission in the striatum and other brain regions involved in motor control .
Pharmacokinetics
- Absorption : Ropinirole is rapidly absorbed after oral administration, reaching peak plasma concentrations within 1 to 2 hours. Its absolute bioavailability ranges from 45% to 55%, indicating significant first-pass metabolism.
- Distribution : The drug exhibits a wide distribution with an apparent volume of distribution of approximately 7.5 L/kg. About 40% of Ropinirole is bound to plasma proteins .
- Metabolism : It is primarily metabolized in the liver via cytochrome P450 enzymes, particularly CYP1A2, producing several metabolites that are also pharmacologically active.
- Elimination : The elimination half-life is approximately 6 hours, allowing for once-daily dosing in many cases .
Parkinson's Disease
In clinical trials assessing Ropinirole's efficacy in PD, significant improvements were noted:
- Unified Parkinson's Disease Rating Scale (UPDRS) : A study showed that after 12 weeks of treatment with an average daily dosage of 7.4 mg, 71% of patients exhibited a clinical response (≥30% improvement in UPDRS motor score) compared to 41% in the placebo group .
- Motor Symptoms : Ropinirole effectively reduced motor symptoms such as tremor and rigidity. In another trial with a mean daily dosage of 15.7 mg, UPDRS scores improved by 22% with Ropinirole versus a decline of 4% with placebo .
Restless Legs Syndrome
Ropinirole has also demonstrated efficacy in treating RLS:
- IRLS Scale : In trials measuring the International Restless Legs Syndrome Study Group rating scale (IRLS), patients receiving Ropinirole showed greater improvements than those on placebo after 12 weeks .
- Quality of Life : Improvements were noted in sleep quality and overall quality-of-life scores among Ropinirole-treated patients compared to controls .
Behavioral Studies
Recent research has highlighted the effects of Ropinirole on cognitive functions such as inhibition:
- A study indicated that acute doses of Ropinirole significantly reduce proactive inhibition without affecting reactive inhibition in healthy participants. This suggests potential implications for impulsivity and decision-making processes influenced by dopaminergic activity .
ROPALS Trial
The ROPALS trial investigated the safety and efficacy of Ropinirole hydrochloride in patients with amyotrophic lateral sclerosis (ALS). Preliminary results indicated that Ropinirole may offer therapeutic benefits beyond its traditional uses, suggesting a broader application for this compound .
Summary Table of Key Findings
| Study/Trial | Condition | Dosage | Outcome |
|---|---|---|---|
| UPDRS Study | Parkinson's Disease | Mean 7.4 mg | 71% response rate (≥30% improvement) |
| Motor Symptoms Study | Parkinson's Disease | Mean 15.7 mg | UPDRS improved by 22% vs. -4% placebo |
| IRLS Study | Restless Legs | Up to 4 mg | Greater improvement vs. placebo |
| ROPALS Trial | ALS | 2-16 mg | Safety and tolerability evaluated |
Properties
IUPAC Name |
4-[2-[propyl(3,3,3-trideuteriopropyl)amino]ethyl]-1,3-dihydroindol-2-one;hydrochloride | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H24N2O.ClH/c1-3-9-18(10-4-2)11-8-13-6-5-7-15-14(13)12-16(19)17-15;/h5-7H,3-4,8-12H2,1-2H3,(H,17,19);1H/i1D3; | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XDXHAEQXIBQUEZ-NIIDSAIPSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCN(CCC)CCC1=C2CC(=O)NC2=CC=C1.Cl | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])CCN(CCC)CCC1=C2CC(=O)NC2=CC=C1.Cl | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H25ClN2O | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
299.85 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details







Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













