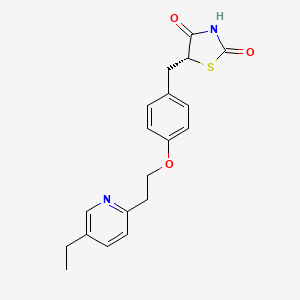
(R)-Pioglitazone
Overview
Description
®-Pioglitazone is a chiral compound and a member of the thiazolidinedione class of drugs. It is primarily used as an antidiabetic agent, specifically for the treatment of type 2 diabetes mellitus. The compound works by improving insulin sensitivity in patients, thereby helping to control blood sugar levels.
Preparation Methods
Synthetic Routes and Reaction Conditions
The synthesis of ®-Pioglitazone involves several steps, starting from the appropriate chiral precursors. One common method includes the use of chiral catalysts to ensure the correct stereochemistry. The reaction conditions typically involve controlled temperatures and pH levels to maintain the integrity of the chiral center.
Industrial Production Methods
In industrial settings, the production of ®-Pioglitazone is scaled up using optimized synthetic routes that ensure high yield and purity. This often involves the use of large-scale reactors and continuous monitoring of reaction conditions to maintain consistency.
Chemical Reactions Analysis
Types of Reactions
®-Pioglitazone undergoes various chemical reactions, including:
Oxidation: This reaction can modify the functional groups on the molecule, potentially altering its activity.
Reduction: Used to convert certain functional groups into more reactive forms.
Substitution: Commonly involves the replacement of one functional group with another, which can be useful in modifying the compound for different applications.
Common Reagents and Conditions
Oxidizing Agents: Such as potassium permanganate or hydrogen peroxide.
Reducing Agents: Like sodium borohydride or lithium aluminum hydride.
Substitution Reagents: Including halogens and nucleophiles.
Major Products
The major products formed from these reactions depend on the specific reagents and conditions used. For example, oxidation might yield hydroxylated derivatives, while substitution could introduce new functional groups.
Scientific Research Applications
Diabetes Management
(R)-Pioglitazone is widely recognized for its efficacy in controlling blood glucose levels in patients with T2DM. It enhances insulin sensitivity and has been shown to lower fasting blood sugar levels significantly. In clinical studies, it has demonstrated:
- Glycemic Control : A meta-analysis indicated that this compound effectively reduces fasting blood sugar levels by an average of 0.24 mmol/L compared to other antidiabetic medications .
- Lipid Profile Improvement : Patients treated with this compound showed significant improvements in triglycerides and HDL cholesterol levels .
Non-Alcoholic Steatohepatitis (NASH)
Recent studies have highlighted the potential of this compound in treating NASH, a common liver condition associated with obesity and diabetes. Research indicates that:
- Liver Health : Preclinical studies demonstrated that deuterium-stabilized this compound (PXL065) significantly reduces hepatic triglycerides, cholesterol, and inflammation .
- Clinical Trials : Phase 1 trials showed that PXL065 is safe and well-tolerated, suggesting its potential as a more effective treatment option compared to traditional pioglitazone formulations.
Kidney Disease Management
This compound has been investigated for its effects on proteinuria reduction in nephrotic syndrome (NS). Key findings include:
- Proteinuria Reduction : In a study involving children with refractory NS, the addition of this compound to glucocorticoid therapy resulted in an 80% reduction in proteinuria .
- Mechanistic Insights : The protective effects were linked to enhanced podocyte function and reduced expression of inflammatory markers, indicating a potential renoprotective role .
Cardiovascular Benefits
The cardiovascular implications of this compound have also been explored:
- Left Ventricular Hypertrophy : Studies show that this compound can improve cardiac hypertrophy associated with hypertension by reducing collagen deposition and myofibroblast proliferation .
- Risk Reduction : Patients with a history of myocardial infarction or stroke who were treated with this compound exhibited a significant reduction in the risk of subsequent cardiovascular events .
Anti-Inflammatory Effects
Research has identified anti-inflammatory properties associated with this compound:
- Chronic Obstructive Pulmonary Disease : In models of chronic obstructive pulmonary disease, this compound demonstrated unique anti-inflammatory effects, suggesting potential applications in respiratory diseases .
- Mechanism Exploration : The anti-inflammatory effects are thought to be mediated through pathways independent of PPARγ activation, which is traditionally linked to thiazolidinediones .
Data Summary
Mechanism of Action
®-Pioglitazone exerts its effects by activating the peroxisome proliferator-activated receptor gamma (PPARγ). This receptor plays a crucial role in the regulation of glucose and lipid metabolism. By binding to PPARγ, ®-Pioglitazone enhances the transcription of insulin-responsive genes, leading to improved insulin sensitivity and glucose uptake in tissues.
Comparison with Similar Compounds
Similar Compounds
Rosiglitazone: Another thiazolidinedione with similar antidiabetic properties.
Troglitazone: An older compound that was withdrawn from the market due to safety concerns.
Ciglitazone: A less commonly used thiazolidinedione.
Uniqueness
®-Pioglitazone is unique in its chiral nature, which can influence its pharmacokinetics and pharmacodynamics. Its specific interaction with PPARγ and the resulting effects on insulin sensitivity make it a valuable option for the treatment of type 2 diabetes.
Properties
CAS No. |
959687-65-1 |
|---|---|
Molecular Formula |
C19H20N2O3S |
Molecular Weight |
356.4 g/mol |
IUPAC Name |
(5R)-5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione |
InChI |
InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23)/t17-/m1/s1 |
InChI Key |
HYAFETHFCAUJAY-QGZVFWFLSA-N |
Isomeric SMILES |
CCC1=CN=C(C=C1)CCOC2=CC=C(C=C2)C[C@@H]3C(=O)NC(=O)S3 |
Canonical SMILES |
CCC1=CN=C(C=C1)CCOC2=CC=C(C=C2)CC3C(=O)NC(=O)S3 |
Origin of Product |
United States |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details






Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.














