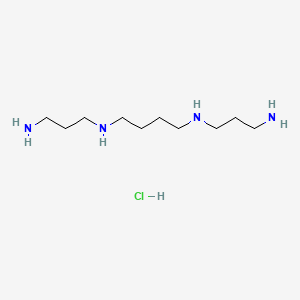
Spermine hydrochloride
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Description
Spermine hydrochloride is a useful research compound. Its molecular formula is C10H27ClN4 and its molecular weight is 238.80 g/mol. The purity is usually 95%.
BenchChem offers high-quality this compound suitable for many research applications. Different packaging options are available to accommodate customers' requirements. Please inquire for more information about this compound including the price, delivery time, and more detailed information at [email protected].
Scientific Research Applications
Molecular Biology and Biochemistry
Spermine hydrochloride plays a crucial role in molecular biology and biochemistry research. Its polycationic nature allows it to interact with nucleic acids, making it valuable for several applications:
- DNA Precipitation and Isolation : Spermine is used to precipitate DNA from low salt aqueous buffers, facilitating the isolation of DNA fragments longer than 100 base pairs. It has been utilized in chromosome isolation and chromatin aggregation .
- Gene Transfer Agents : The compound serves as a building block for preparing gene transfer agents. Its ability to complex with DNA forms nanoparticles with diameters less than 100 nm, enhancing gene delivery systems .
- Crystallization of Nucleic Acids : Spermine has been successfully employed in the crystallization of DNA, which is essential for structural studies of nucleic acids .
- MALDI-MS Matrix : It acts as a matrix in Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS), aiding in the analysis of glycoconjugates and oligonucleotides .
Neurobiology
This compound exhibits neuroprotective properties, particularly concerning its interaction with neurotransmitter receptors:
- NMDA Receptor Modulation : Spermine has been shown to inhibit the N-methyl-D-aspartate (NMDA) receptor and voltage-activated calcium channels. This inhibition is significant for neuroprotection in acute hippocampal slices .
- Cytokine Inhibition : It suppresses pro-inflammatory cytokine synthesis in human peripheral blood mononuclear cells (PBMCs). Studies indicate that spermine can inhibit tumor necrosis factor (TNF) synthesis induced by lipopolysaccharides (LPS), suggesting potential therapeutic applications in inflammatory conditions .
Cancer Research
The compound's role extends into cancer research, where it has been implicated in modulating cellular processes:
- Autophagy Regulation : Spermine influences autophagy pathways, which are crucial for maintaining cellular homeostasis and preventing cancer cell proliferation. By mimicking caloric restriction, spermine enhances autophagic activity, potentially improving chemotherapy efficacy .
- Lifespan Extension Studies : Research indicates that spermine supplementation can prolong lifespan in model organisms by activating autophagy and reducing oxidative stress, which are critical factors in cancer development .
Dermatological Applications
Recent studies have explored the potential of this compound in dermatology:
- Melanogenesis Enhancement : Spermine has been found to increase melanin production in melanocytes, suggesting its utility as a pigmenting agent for treating hypopigmentation disorders . This effect is mediated through stabilization of tyrosinase-related proteins involved in melanin synthesis.
Case Studies and Data Tables
The following table summarizes key findings from various studies on the applications of this compound:
Properties
CAS No. |
29605-46-7 |
|---|---|
Molecular Formula |
C10H27ClN4 |
Molecular Weight |
238.80 g/mol |
IUPAC Name |
N,N'-bis(3-aminopropyl)butane-1,4-diamine;hydrochloride |
InChI |
InChI=1S/C10H26N4.ClH/c11-5-3-9-13-7-1-2-8-14-10-4-6-12;/h13-14H,1-12H2;1H |
InChI Key |
KBDDIZRDKLGWGW-UHFFFAOYSA-N |
Canonical SMILES |
C(CCNCCCN)CNCCCN.Cl |
physical_description |
Solid; [Merck Index] White hygroscopic powder; [Sigma-Aldrich MSDS] |
Origin of Product |
United States |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















