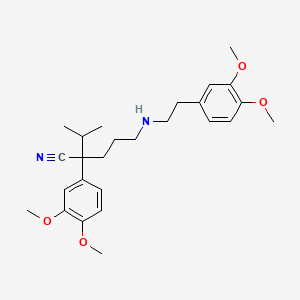
Norverapamil
Overview
Description
Norverapamil is a calcium channel blocker and the primary active metabolite of verapamil. It is known for its significant role in cardiovascular treatments, particularly in managing conditions such as hypertension, angina, and certain types of arrhythmias . The compound’s chemical formula is C26H36N2O4, and it has a molar mass of 440.58 g/mol .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of norverapamil typically involves the demethylation of verapamil. One common method includes reacting verapamil with 1-chloroethyl chloroformate in an aprotic solvent. The reaction is carried out at temperatures ranging from 10°C to 80°C, resulting in the formation of a quaternary ammonium salt. This intermediate is then subjected to further reaction to remove the methyl group, yielding this compound .
Industrial Production Methods: Industrial production of this compound follows similar synthetic routes but on a larger scale. The process is optimized for high conversion rates and ease of separation. The use of cost-effective raw materials and controlled reaction conditions ensures efficient production .
Chemical Reactions Analysis
Types of Reactions: Norverapamil undergoes various chemical reactions, including:
Reduction: Involves the removal of oxygen or the addition of hydrogen, typically using reducing agents.
Substitution: Involves the replacement of one functional group with another, often using nucleophiles or electrophiles.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include potassium permanganate and chromium trioxide.
Reduction: Common reducing agents include lithium aluminum hydride and sodium borohydride.
Substitution: Common reagents include halogens and alkylating agents.
Major Products: The major products formed from these reactions depend on the specific conditions and reagents used. For example, oxidation may yield hydroxylated derivatives, while reduction may produce deoxygenated compounds .
Scientific Research Applications
Pharmacological Properties
Norverapamil exhibits notable pharmacological properties that make it a candidate for addressing several medical challenges:
- Multidrug Resistance Reversal : this compound has been shown to be more effective than verapamil in reversing multidrug resistance (MDR) in cancer therapy. It operates by inhibiting P-glycoprotein, a key player in drug efflux mechanisms that contribute to MDR. Compared to verapamil, this compound has significantly reduced cardiotoxicity, making it a safer alternative for patients undergoing chemotherapy .
- Antitubercular Activity : Recent studies indicate that this compound can inhibit macrophage-induced tolerance to multiple anti-tubercular drugs. It has been demonstrated to reduce intracellular growth of Mycobacterium tuberculosis, suggesting its potential role in shortening tuberculosis treatment regimens .
Synthesis and Production
The synthesis of this compound remains a critical area of research due to its therapeutic potential:
- Biocatalytic Methods : Innovative approaches using cytochrome P450 enzymes have been developed for the efficient production of this compound. A study highlighted the use of CYP105D1 from Streptomyces griseus for selective N-demethylation of verapamil, resulting in high yields under mild conditions. This method is anticipated to enhance the production capabilities within the pharmaceutical industry .
Clinical Applications
This compound's clinical relevance is underscored by its pharmacokinetic properties and therapeutic efficacy:
- Pediatric Use : A study measuring plasma levels of verapamil and this compound in children revealed important insights into their pharmacokinetics. The findings indicated variability in drug concentrations based on age and dosage, highlighting the need for tailored dosing strategies in pediatric populations .
- Drug Interaction Studies : this compound has been included in pharmacokinetic models assessing drug-drug interactions (DDIs). Its interaction with various medications has been studied to predict potential adverse effects and optimize therapeutic regimens .
Data Table: Summary of this compound Applications
Case Study 1: Multidrug Resistance Reversal
In a controlled study, this compound was administered alongside standard chemotherapy agents to evaluate its efficacy in reversing MDR. Results demonstrated a significant reduction in tumor cell viability compared to controls treated with chemotherapy alone.
Case Study 2: Tuberculosis Treatment
A clinical trial involving patients with drug-resistant tuberculosis assessed the impact of adding this compound to standard treatment regimens. The trial reported improved outcomes with reduced treatment duration and enhanced tolerability.
Mechanism of Action
Norverapamil exerts its effects by inhibiting the influx of calcium ions through L-type calcium channels in the cell membrane. This inhibition leads to the relaxation of vascular smooth muscle, resulting in vasodilation and reduced blood pressure. The compound also affects the conduction of electrical impulses in the heart, helping to manage arrhythmias. The primary molecular targets are the calcium channels in the cardiovascular system .
Comparison with Similar Compounds
Verapamil: The parent compound of norverapamil, also a calcium channel blocker with similar therapeutic uses.
Diltiazem: Another calcium channel blocker used to treat hypertension and angina.
Nifedipine: A dihydropyridine calcium channel blocker used primarily for hypertension.
Uniqueness of this compound: this compound is unique due to its specific metabolic origin from verapamil and its distinct pharmacokinetic properties. It has a longer half-life and different metabolic pathways compared to its parent compound, making it particularly effective in certain therapeutic contexts .
Biological Activity
Norverapamil, the N-demethylated metabolite of verapamil, has garnered attention in pharmacological research due to its distinct biological activities, particularly in the context of drug metabolism, cardiovascular effects, and potential therapeutic applications. This article synthesizes findings from diverse studies to provide a comprehensive overview of this compound's biological activity.
This compound is formed through the N-demethylation of verapamil, primarily mediated by cytochrome P450 enzymes. The CYP105D1 enzyme from Streptomyces griseus has been identified as capable of efficiently catalyzing this reaction, achieving a bioconversion rate of 60.16% within 24 hours under optimized conditions . This enzymatic process highlights the potential for biotechnological applications in producing this compound.
Pharmacokinetics
The pharmacokinetics of this compound reveal significant interindividual variability. In a study involving children treated with verapamil for supraventricular tachyarrhythmias, trough plasma levels of this compound averaged 41.7 ng/ml, correlating with the daily dose . The ratio of this compound to verapamil concentrations (N/V ratio) indicated metabolic clearance improved with age, suggesting developmental factors influence drug metabolism .
| Parameter | Mean Value | Standard Deviation |
|---|---|---|
| Verapamil Trough Level | 43.3 ng/ml | 36.4 |
| This compound Trough Level | 41.7 ng/ml | 28.9 |
| N/V Ratio | Variable by Age | - |
Cardiovascular Effects
While this compound exhibits less potency than verapamil as a calcium channel blocker, it retains significant biological activity. Research indicates that this compound can modulate cardiac function and may contribute to the therapeutic effects observed with verapamil . In particular, its role in managing supraventricular tachyarrhythmias has been documented, albeit with caution due to its lower efficacy compared to its parent compound.
Antimicrobial Properties
Recent studies have highlighted this compound's potential in combating Mycobacterium tuberculosis. Both verapamil and this compound were shown to inhibit intracellular growth and drug tolerance mechanisms in macrophage-like cells infected with M. tuberculosis. Notably, this compound was effective at lower concentrations than those required for verapamil . This suggests that this compound may serve as a valuable adjunct in tuberculosis treatment regimens.
Clinical Studies and Case Reports
A dose-finding study evaluated the pharmacokinetics of this compound alongside verapamil in participants receiving rifampin. The study found that higher doses of verapamil resulted in increased levels of both compounds, with a notable increase in the AUC (area under the curve) for this compound compared to baseline measurements . The findings support the hypothesis that drug-drug interactions can significantly alter the pharmacokinetic profiles of these compounds.
Adverse Effects and Safety Profile
Adverse reactions associated with this compound are similar to those seen with verapamil but generally occur at lower frequencies. In pediatric populations, common side effects included bradycardia and hypotension, particularly when plasma levels exceeded therapeutic thresholds . Monitoring is essential to mitigate risks associated with elevated plasma concentrations.
Properties
IUPAC Name |
2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethylamino]-2-propan-2-ylpentanenitrile | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C26H36N2O4/c1-19(2)26(18-27,21-9-11-23(30-4)25(17-21)32-6)13-7-14-28-15-12-20-8-10-22(29-3)24(16-20)31-5/h8-11,16-17,19,28H,7,12-15H2,1-6H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UPKQNCPKPOLASS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(CCCNCCC1=CC(=C(C=C1)OC)OC)(C#N)C2=CC(=C(C=C2)OC)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C26H36N2O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
67812-42-4 (mono-hydrochloride) | |
| Record name | Norverapamil | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0067018853 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID80873799 | |
| Record name | Norverapamil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80873799 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
440.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
67018-85-3 | |
| Record name | Norverapamil | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=67018-85-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Norverapamil | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0067018853 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Norverapamil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80873799 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 5-[(3,4-dimethoxyphenethyl)amino]-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.060.476 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | NORVERAPAMIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/957Z3K3R56 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













