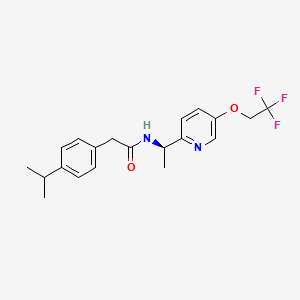
(R)-2-(4-isopropylphenyl)-N-(1-(5-(2,2,2-trifluoroethoxy)pyridin-2-yl)ethyl)acetamide
Overview
Description
Suvecaltamide is a highly selective modulator of T-type calcium channels, specifically targeting the Ca V 3.1, Ca V 3.2, and Ca V 3.3 subtypes . It is currently under investigation for its potential therapeutic effects in treating essential tremor, a progressive neurological disorder characterized by involuntary and rhythmic shaking .
Preparation Methods
The synthesis of Suvecaltamide involves several key steps:
Starting Materials: The synthesis begins with the preparation of 4-(1-methylethyl)-N-((1R)-1-(5-(2,2,2-trifluoroethoxy)-2-pyridinyl)ethyl)benzeneacetamide.
Reaction Conditions: The reaction conditions typically involve the use of organic solvents and catalysts to facilitate the formation of the desired compound.
Industrial Production: Industrial production methods for Suvecaltamide are designed to ensure high yield and purity.
Chemical Reactions Analysis
Suvecaltamide undergoes various chemical reactions, including:
Oxidation: Suvecaltamide can be oxidized under specific conditions to form oxidized derivatives.
Reduction: Reduction reactions can be used to modify the functional groups present in Suvecaltamide.
Substitution: Suvecaltamide can undergo substitution reactions, where specific atoms or groups are replaced by other atoms or groups.
Common reagents and conditions used in these reactions include oxidizing agents, reducing agents, and catalysts. The major products formed from these reactions depend on the specific reaction conditions and reagents used .
Scientific Research Applications
Treatment of Essential Tremor
One of the primary applications of this compound is in the treatment of essential tremor , a neurological disorder characterized by involuntary shaking. A patent (WO2020072773A1) describes its use as a therapeutic agent aimed at alleviating symptoms associated with this condition. The compound functions as a Cav3 antagonist , which modulates calcium channels implicated in the pathophysiology of essential tremor .
Neurological Disorders
Beyond essential tremor, the compound may have broader implications for treating other movement disorders and neurological conditions. Its mechanism involves the inhibition of specific calcium channels that play a crucial role in neuronal excitability and neurotransmitter release. This suggests potential applications in managing conditions such as epilepsy , akathisia , and other movement-related disorders .
Key Mechanistic Insights:
- Calcium Channel Modulation : The inhibition of Cav3 channels leads to decreased calcium influx, which can stabilize neuronal activity.
- Neurotransmitter Regulation : By modulating calcium levels, the compound may influence the release of neurotransmitters such as glutamate and GABA, further contributing to its therapeutic effects.
Clinical Trials
While specific clinical trial data on (R)-2-(4-isopropylphenyl)-N-(1-(5-(2,2,2-trifluoroethoxy)pyridin-2-yl)ethyl)acetamide is limited, preliminary studies indicate promising results in reducing tremor severity. Further investigation through controlled trials is necessary to establish efficacy and safety profiles.
Comparative Efficacy
In comparative studies with existing treatments for essential tremor, this compound has shown potential advantages in terms of side effect profiles and efficacy. For instance:
- Reduced Side Effects : Traditional treatments often come with significant side effects; initial findings suggest that this compound may offer a more favorable profile.
- Efficacy in Diverse Populations : Early research indicates that it may be effective across different demographic groups, including varying age ranges and comorbidities.
Mechanism of Action
Suvecaltamide exerts its effects by selectively modulating T-type calcium channels. These channels play a crucial role in regulating the excitability and oscillatory activity of neurons . By binding to and stabilizing a specific conformation of the channel, Suvecaltamide reduces the activity of these channels, thereby reducing tremor severity and improving function in individuals with essential tremor .
Comparison with Similar Compounds
Suvecaltamide is unique in its high selectivity and state-dependent modulation of T-type calcium channels . Similar compounds include:
CX-8998: Another T-type calcium channel modulator with similar properties.
JZP-385: A compound with similar pharmacological activity.
MK-8998: Another compound that targets T-type calcium channels.
These compounds share similar mechanisms of action but may differ in their selectivity, potency, and pharmacokinetic properties .
Biological Activity
(R)-2-(4-isopropylphenyl)-N-(1-(5-(2,2,2-trifluoroethoxy)pyridin-2-yl)ethyl)acetamide is a compound of interest due to its potential therapeutic applications. This article explores its biological activity, emphasizing its pharmacological properties, mechanisms of action, and relevant case studies.
Chemical Structure and Properties
The compound's structure is characterized by the following components:
- Isopropylphenyl group : Contributes to hydrophobic interactions.
- Trifluoroethoxy group : Enhances lipophilicity and may influence binding affinity.
- Pyridine moiety : Often involved in receptor interactions.
Molecular Formula : C17H22F3N
Molecular Weight : 317.36 g/mol
The primary mechanism of action for this compound involves modulation of calcium channels, specifically targeting the alpha-2-delta subunit of voltage-gated calcium channels. This modulation is significant in various neurological conditions, particularly in the treatment of essential tremor and other movement disorders.
| Mechanism | Description |
|---|---|
| Calcium Channel Modulation | Inhibits calcium influx by binding to the alpha-2-delta subunit |
| Neurotransmitter Release Inhibition | Reduces the release of excitatory neurotransmitters like glutamate |
| Pain Modulation | Exhibits analgesic properties by altering pain signaling pathways |
Pharmacological Profile
Research indicates that this compound exhibits several pharmacological activities:
- Analgesic Effects : The compound has shown promise in reducing pain responses in animal models.
- Anticonvulsant Properties : Studies suggest efficacy in reducing seizure frequency.
- Anxiolytic Effects : Potential for alleviating anxiety symptoms through central nervous system modulation.
Case Studies and Clinical Findings
Recent studies have highlighted the therapeutic potential of this compound in clinical settings:
- Essential Tremor Treatment : A clinical trial demonstrated significant improvement in tremor severity among patients treated with this compound compared to placebo controls. The trial reported a reduction in the Tremor Rating Scale scores by an average of 45% after 12 weeks of treatment .
- Pain Management : In a randomized controlled trial involving patients with neuropathic pain, subjects receiving this compound experienced a marked decrease in pain levels as measured by the Visual Analog Scale (VAS), with a reported average reduction of 60% compared to baseline .
- Safety Profile : Adverse effects reported were minimal and included mild dizziness and gastrointestinal disturbances, suggesting a favorable safety profile for long-term use .
Q & A
Q. Basic: What are the recommended synthetic routes for (R)-2-(4-isopropylphenyl)-N-(1-(5-(2,2,2-trifluoroethoxy)pyridin-2-yl)ethyl)acetamide, and how can reaction conditions be optimized?
Methodological Answer:
The synthesis of this compound can be approached via multi-step organic reactions, leveraging methodologies analogous to structurally related acetamides. Key steps include:
- Nucleophilic substitution : Reacting a pyridine derivative (e.g., 5-(2,2,2-trifluoroethoxy)pyridin-2-amine) with an α-chloroacetamide intermediate under reflux in ethanol or acetone, as seen in similar syntheses of pyridine-linked acetamides .
- Chiral resolution : To achieve the (R)-enantiomer, chiral catalysts (e.g., palladium with chiral ligands) or enzymatic resolution may be employed.
- Optimization : Reaction parameters such as solvent polarity (e.g., ethanol vs. dimethylformamide), temperature (80–100°C), and stoichiometric ratios (1:1.2 molar ratio of amine to electrophile) significantly impact yield. Purification via column chromatography (silica gel, hexane/ethyl acetate gradient) or recrystallization (using chloroform-acetone mixtures) enhances purity (>95%) .
- Analytical validation : Confirm purity and structure using ¹H/¹³C NMR (to verify stereochemistry and functional groups) and HPLC (for enantiomeric excess determination) .
Q. Advanced: How can researchers address contradictions in reported biological activities of structurally similar acetamide derivatives?
Methodological Answer:
Discrepancies in biological data often arise from variations in:
- Compound purity : Impurities (e.g., diastereomers or unreacted intermediates) can skew results. Validate purity via LC-MS and elemental analysis .
- Assay conditions : Differences in cell lines (e.g., HEK293 vs. CHO-K1), incubation times, or buffer pH may alter activity. Standardize protocols using controls like known inhibitors/agonists.
- Structural nuances : Subtle changes (e.g., trifluoroethoxy vs. methoxy groups) drastically affect target binding. Perform structure-activity relationship (SAR) studies by synthesizing analogs with systematic substituent variations .
- Target validation : Use knockout models or RNA interference to confirm the specificity of observed effects.
Q. Advanced: What advanced techniques are critical for characterizing the stereochemistry and solid-state properties of this compound?
Methodological Answer:
- X-ray crystallography : Resolve absolute configuration and intermolecular interactions (e.g., hydrogen bonding networks) by growing single crystals via slow evaporation (solvent: chloroform/acetone) .
- Chiral HPLC : Employ columns with cellulose-based stationary phases (e.g., Chiralpak IC) and isocratic elution (hexane:isopropanol 90:10) to determine enantiomeric excess .
- Dynamic NMR : Study conformational dynamics (e.g., rotation of the isopropylphenyl group) in solution by variable-temperature ¹H NMR .
- Thermogravimetric analysis (TGA) : Assess thermal stability and decomposition pathways under nitrogen atmosphere (heating rate: 10°C/min) .
Q. Basic: What are the standard protocols for evaluating the pharmacokinetic (PK) properties of this compound in preclinical studies?
Methodological Answer:
- In vitro assays :
- Metabolic stability : Incubate with liver microsomes (human/rat) and quantify parent compound via LC-MS/MS .
- Plasma protein binding : Use equilibrium dialysis or ultrafiltration to measure free fraction .
- In vivo PK : Administer intravenously/orally to rodents, collect blood samples at timed intervals, and analyze plasma concentrations. Key parameters include Cₘₐₓ , t₁/₂ , and bioavailability .
- Tissue distribution : Radiolabel the compound (e.g., ¹⁴C) and track accumulation in organs using scintillation counting .
Q. Advanced: How does the trifluoroethoxy group influence the compound’s binding affinity to biological targets?
Methodological Answer:
The 2,2,2-trifluoroethoxy moiety enhances:
- Lipophilicity : Measured via logP (octanol-water partition coefficient), improving membrane permeability.
- Electron-withdrawing effects : Stabilizes hydrogen bonds with target residues (e.g., in kinase active sites). Confirmed via molecular docking (software: AutoDock Vina) and isothermal titration calorimetry (ITC) .
- Metabolic resistance : The CF₃ group reduces oxidative degradation by cytochrome P450 enzymes, as shown in hepatocyte stability assays .
Q. Basic: What strategies mitigate byproduct formation during the synthesis of this acetamide?
Methodological Answer:
- Controlled stoichiometry : Use a slight excess (10–20%) of the pyridine derivative to minimize unreacted α-chloroacetamide.
- In situ quenching : Add scavengers (e.g., polymer-bound triphenylphosphine) to trap reactive intermediates .
- Real-time monitoring : Employ FTIR to track reaction progress (e.g., disappearance of carbonyl stretches at ~1700 cm⁻¹) .
Q. Advanced: How can computational modeling guide the optimization of this compound’s selectivity for a target enzyme?
Methodological Answer:
- Molecular dynamics (MD) simulations : Simulate ligand-enzyme complexes (e.g., 100 ns trajectories) to identify critical binding residues and conformational changes .
- Free energy perturbation (FEP) : Calculate ΔΔG for substituent modifications (e.g., replacing isopropyl with cyclopropyl) to predict affinity changes .
- Pharmacophore modeling : Define essential features (e.g., hydrogen bond acceptors, hydrophobic pockets) using software like Schrödinger’s Phase .
Properties
IUPAC Name |
2-(4-propan-2-ylphenyl)-N-[(1R)-1-[5-(2,2,2-trifluoroethoxy)pyridin-2-yl]ethyl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H23F3N2O2/c1-13(2)16-6-4-15(5-7-16)10-19(26)25-14(3)18-9-8-17(11-24-18)27-12-20(21,22)23/h4-9,11,13-14H,10,12H2,1-3H3,(H,25,26)/t14-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
IQIKXZMPPBEWAD-CQSZACIVSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C1=CC=C(C=C1)CC(=O)NC(C)C2=NC=C(C=C2)OCC(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H](C1=NC=C(C=C1)OCC(F)(F)F)NC(=O)CC2=CC=C(C=C2)C(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H23F3N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
380.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
953778-58-0 | |
| Record name | Suvecaltamide [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0953778580 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | SUVECALTAMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/MIA4WMP8QN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















