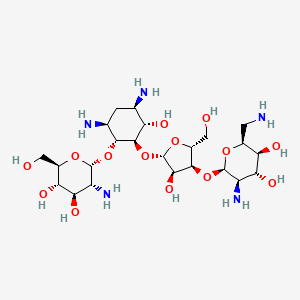
Paromomycin
Overview
Description
Paromomycin is an aminoglycoside antibiotic that is primarily used to treat various parasitic infections, including amebiasis, giardiasis, leishmaniasis, and tapeworm infections . It was discovered in the 1950s from a type of Streptomyces and came into medical use in 1960 . This compound is on the World Health Organization’s List of Essential Medicines .
Preparation Methods
Paromomycin is produced by the bacterium Streptomyces rimosus var. paromomycinus . The industrial production of this compound typically involves fermentation processes. Solid-state fermentation has been shown to be a promising method for producing this compound, offering advantages such as cost reduction of raw materials, less energy consumption, and reduced wastewater discharge . The optimization of environmental conditions, such as pH, temperature, and inoculum size, can significantly enhance the yield of this compound .
Chemical Reactions Analysis
Paromomycin undergoes various chemical reactions, including oxidation and substitution reactions. The compound contains oxidizable groups such as amines and hydroxyls, which can be detected electrochemically . Common reagents used in these reactions include oxidizing agents and derivatization agents for detection purposes. The major products formed from these reactions depend on the specific conditions and reagents used.
Scientific Research Applications
Therapeutic Use in Parasitic Infections
Leishmaniasis Treatment
Paromomycin has been extensively studied for its efficacy against leishmaniasis, a disease caused by protozoan parasites transmitted by sandflies. It is particularly noted for its effectiveness in both cutaneous and visceral forms of the disease.
- Cutaneous Leishmaniasis : A meta-analysis revealed that this compound treatment had a success rate approximately 2.79 times higher than that of placebo treatments . In a study involving topical applications, this compound demonstrated a cure rate close to 80% against Leishmania panamensis species .
- Visceral Leishmaniasis : A Phase IIIb clinical trial assessed intramuscular injections of this compound at 11 mg/kg for 21 days in Bangladeshi patients. The results indicated significant clinical responses, with many patients showing resolution of symptoms and no new signs six months post-treatment .
| Study Type | Population | Treatment Duration | Success Rate (%) | Reference |
|---|---|---|---|---|
| Meta-analysis | Various | N/A | 2.79 times placebo | |
| Topical application | CL patients | Varies | ~80% | |
| IM Injection | VL patients | 21 days | N/A |
Research on Glioblastoma
Recent studies have explored the potential of this compound as a therapeutic agent for glioblastoma, a highly aggressive brain tumor. A study published in December 2024 investigated its effects on SUMOylation pathways mediated by histone deacetylase 1 (HDAC1) in glioblastoma cells.
- Mechanism of Action : this compound was identified as a potential HDAC1 inhibitor through molecular docking analysis. In vitro assays showed that it significantly reduced cell viability and migration in glioblastoma cells while modulating SUMO1 expression and decreasing IGF1R nuclear translocation .
- Clinical Implications : These findings suggest that this compound may offer a novel approach to glioblastoma treatment by targeting specific molecular pathways, warranting further clinical investigation.
Broader Applications
Beyond its use in treating leishmaniasis and glioblastoma, this compound has been investigated for other conditions:
- Amebiasis : this compound is effective against Entamoeba histolytica, the causative agent of amebic dysentery, providing an alternative to more toxic treatments such as metronidazole .
- Cryptosporidiosis : This compound has also shown promise against Cryptosporidium infections, particularly in immunocompromised patients, due to its low toxicity profile compared to traditional therapies .
Mechanism of Action
Paromomycin exerts its effects by inhibiting protein synthesis in bacteria. It binds to the 16S ribosomal RNA within the 30S ribosomal subunit, causing misreading of the genetic code and preventing the formation of functional proteins . This leads to bacterial cell death. The molecular targets involved in this process include the ribosomal RNA and the ribosomal subunits .
Comparison with Similar Compounds
Paromomycin is similar to other aminoglycoside antibiotics such as neomycin and streptomycin . it has unique properties that make it effective against a broader range of parasitic infections. Unlike some other aminoglycosides, this compound is poorly absorbed in the gastrointestinal tract, making it particularly useful for treating intestinal infections . Additionally, this compound has been shown to have fewer systemic side effects compared to pentavalent antimony compounds used in the treatment of leishmaniasis .
Similar Compounds
- Neomycin
- Streptomycin
- Gentamicin
- Tobramycin
This compound’s unique properties and broad-spectrum activity make it a valuable antibiotic in both clinical and research settings.
Biological Activity
Paromomycin is an aminoglycoside antibiotic primarily recognized for its effectiveness against various parasitic infections, particularly those caused by Leishmania and Cryptosporidium. Its mechanism of action primarily involves the inhibition of protein synthesis by binding to the ribosomal RNA of target organisms. This article explores the biological activity of this compound, highlighting its therapeutic applications, mechanisms, and clinical findings.
This compound exerts its antimicrobial effects by binding to the 16S ribosomal RNA component of the 30S ribosomal subunit in bacteria and protozoa. This binding leads to misreading of mRNA and the production of defective polypeptides, ultimately resulting in cell death. The antibiotic demonstrates a differential affinity for ribosomal components between protozoan and mammalian cells, which underlies its selective toxicity:
- Protozoan Ribosomes : this compound binds strongly, inhibiting protein synthesis significantly.
- Mammalian Ribosomes : The interaction is minimal, leading to little effect on mammalian protein synthesis .
Efficacy Against Leishmaniasis
Recent studies have demonstrated this compound's effectiveness in treating cutaneous leishmaniasis. In a clinical trial conducted in Iran, this compound showed a success rate of treatment that was 2.79 times higher than that of placebo treatments. The meta-analysis indicated that this compound was significantly more effective compared to other interventions like photodynamic therapy (PDT) and intralesional meglumine antimoniate (MA) .
Table 1: Success Rates of this compound in Treating Leishmaniasis
Clinical Applications
This compound is used not only for leishmaniasis but also as a treatment for intestinal amebiasis and as an adjunct therapy for hepatic encephalopathy. Its administration can be oral or intramuscular, with intramuscular injections being particularly effective in visceral leishmaniasis (VL) cases.
In a Phase IIIb trial in Bangladesh, this compound administered at a dose of 11 mg/kg intramuscularly once daily for 21 days proved effective against VL, demonstrating significant clinical improvement in patients .
Case Studies
- Topical Application : A study involving topical application of a 15% this compound formulation showed a cure rate of 77.5% among patients with Leishmania braziliensis cutaneous leishmaniasis .
- Intramuscular Efficacy : Another investigation highlighted that PMIM (this compound IM Injection) was effective and safe for treating VL, with a notable resolution of symptoms at the end of treatment and sustained clinical response six months post-treatment .
- Cryptosporidiosis : In patients with AIDS suffering from cryptosporidiosis, this compound exhibited modest activity, offering some therapeutic benefit where other treatments failed .
Safety Profile
While generally well-tolerated, this compound can cause side effects such as nephrotoxicity and ototoxicity, particularly at higher doses or prolonged use. Monitoring is essential during treatment to mitigate potential adverse effects .
Q & A
Basic Research Questions
Q. What are the key pharmacokinetic (PK) parameters of paromomycin, and how do they influence dosing regimens in heterogeneous patient populations?
Answer: this compound’s PK parameters (e.g., clearance, volume of distribution) vary across populations due to factors like age, renal function, and comorbidities. A population pharmacokinetic approach is recommended to analyze sparse or heterogeneous data, as demonstrated in pooled analyses integrating trials with differing dosing regimens (e.g., 15 mg/kg/day vs. 20 mg/kg/day) and companion drugs (e.g., miltefosine) . Covariates such as serum creatinine and albumin levels should be prioritized to explain inter-individual variability .
Q. How is this compound’s efficacy evaluated in visceral leishmaniasis (VL) clinical trials, and what are common confounding factors in outcome measurement?
Answer: Efficacy is measured via parasite clearance rates and relapse-free survival over 6–12 months. Confounding factors include geographic variations in parasite resistance, baseline immune status (e.g., albumin/neutrophil levels), and inconsistent PK sampling (e.g., trough samples taken post-dose). Exclusion criteria for unreliable PK data (e.g., Day 1 timing errors) must be predefined to avoid bias .
Q. What methodological considerations are critical when designing combination therapy studies involving this compound?
Answer: Use a PICOT framework to define:
- P opulation: Pediatric vs. adult VL patients.
- I ntervention: this compound + miltefosine vs. monotherapy.
- C omparison: Historical controls or placebo (if ethical).
- O utcome: Cure rate at 6 months, stratified by covariates (e.g., renal function).
- T ime: Follow-up duration aligned with relapse patterns .
Advanced Research Questions
Q. How can population pharmacokinetic/pharmacodynamic (PK/PD) modeling address this compound’s variable exposure-response relationships?
Answer: Nonlinear mixed-effects models (e.g., NONMEM) are essential to handle sparse sampling and heterogeneous data. For example, Ethiopian trials with sparse PK samples required covariate adjustments (e.g., serum creatinine) to explain geographic variability in drug exposure. Exclude implausible data (e.g., TAD=0/24h samples with high variability) to refine parameter estimates .
Q. What mechanisms underlie this compound resistance in Leishmania spp., and how can in vitro assays inform clinical resistance monitoring?
Answer: Resistance is linked to ribosomal RNA mutations and reduced drug uptake. Use in vitro susceptibility assays (e.g., promastigote/amastigote IC50) combined with genomic sequencing of clinical isolates. Correlate in vitro findings with clinical failure rates, adjusting for covariates like treatment duration and PK variability .
Q. How do covariates like renal impairment or malnutrition impact this compound’s safety profile, and how should these be analyzed in retrospective studies?
Answer: Renal impairment prolongs drug exposure, increasing nephrotoxicity risk. Use multivariate regression to analyze longitudinal serum creatinine and albumin data. In Indian cohorts, missing creatinine data necessitated sensitivity analyses to validate findings .
Q. What statistical methods resolve contradictions in this compound efficacy data across regions (e.g., South Asia vs. East Africa)?
Answer: Meta-regression models can adjust for confounders like parasite strain differences, baseline immunity, and dosing adherence. Stratify data by region and apply bootstrapping to assess robustness of efficacy estimates .
Q. How can in vitro-in vivo correlation (IVIVC) models optimize this compound dosing for emerging pathogens?
Answer: Develop IVIVC using time-kill curves and PK/PD indices (e.g., AUC/MIC). Validate with hollow-fiber infection models simulating human PK profiles. Prioritize pathogens with zoonotic potential (e.g., Cryptosporidium) where this compound shows off-label promise .
Q. What systematic review strategies are effective for synthesizing this compound’s safety data across fragmented clinical trials?
Answer: Follow PRISMA guidelines with strict inclusion criteria (e.g., RCTs, cohort studies ≥50 patients). Extract adverse event data using MedDRA coding, and assess quality via GRADE criteria. Use forest plots to visualize heterogeneity in toxicity rates (e.g., ototoxicity in pediatric vs. adult cohorts) .
Q. How should ethical challenges in this compound trials (e.g., pediatric dosing, placebo use) be addressed in study protocols?
Answer: For pediatric trials, use adaptive designs with Bayesian dose escalation to minimize toxicity. Justify placebo arms with historical control data and obtain ethics committee approval via detailed risk-benefit analyses. Include DSMB oversight for early termination rules .
Q. Tables for Key Evidence Synthesis
| Parameter | Findings | Source |
|---|---|---|
| PK Variability | 27.2% of trough samples excluded due to implausible TAD=0/24h data | |
| Covariate Impact | Serum creatinine and albumin explained 40% of PK variability in East Africa | |
| Resistance Mechanisms | Leishmania rRNA mutations reduce drug binding affinity by 60% |
Properties
IUPAC Name |
(2S,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(2R,3S,4R,5S)-5-[(1R,2R,3S,5R,6S)-3,5-diamino-2-[(2S,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-hydroxycyclohexyl]oxy-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxyoxane-3,4-diol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H45N5O14/c24-2-7-13(32)15(34)10(27)21(37-7)41-19-9(4-30)39-23(17(19)36)42-20-12(31)5(25)1-6(26)18(20)40-22-11(28)16(35)14(33)8(3-29)38-22/h5-23,29-36H,1-4,24-28H2/t5-,6+,7+,8-,9-,10-,11-,12+,13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
UOZODPSAJZTQNH-LSWIJEOBSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1C(C(C(C(C1N)OC2C(C(C(C(O2)CO)O)O)N)OC3C(C(C(O3)CO)OC4C(C(C(C(O4)CN)O)O)N)O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1[C@H]([C@@H]([C@H]([C@@H]([C@H]1N)O[C@@H]2[C@@H]([C@H]([C@@H]([C@H](O2)CO)O)O)N)O[C@H]3[C@@H]([C@@H]([C@H](O3)CO)O[C@@H]4[C@@H]([C@H]([C@@H]([C@@H](O4)CN)O)O)N)O)O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H45N5O14 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
1263-89-4 (sulfate), 35665-49-7 (sulfate (2:5)), 7205-49-4 (sulfate (1:1)) | |
| Record name | Paromomycin [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007542372 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8023424 | |
| Record name | Paromomycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023424 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
615.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Paromomycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015490 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
7.97e+01 g/L | |
| Record name | Paromomycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015490 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Paromomycin inhibits protein synthesis by binding to 16S ribosomal RNA. Bacterial proteins are synthesized by ribosomal RNA complexes which are composed of 2 subunits, a large subunit (50s) and small (30s) subunit, which forms a 70s ribosomal subunit. tRNA binds to the top of this ribosomal structure. Paramomycin binds to the A site, which causes defective polypeptide chains to be produced. Continuous production of defective proteins eventually leads to bacterial death. | |
| Record name | Paromomycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01421 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
7542-37-2, 1263-89-4 | |
| Record name | Paromomycin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=7542-37-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Paromomycin [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007542372 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Paromomycin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01421 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Paromomycin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023424 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Paromomycin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.028.567 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PAROMOMYCIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/61JJC8N5ZK | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Paromomycin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015490 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.
















