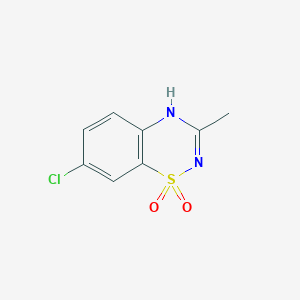
Diazoxide
Overview
Description
Diazoxide is a non-diuretic benzothiadiazine derivative that is primarily used to manage hypoglycemia due to hyperinsulinism. It is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and lacks chloriuretic or natriuretic activity . This compound is known for its ability to activate ATP-sensitive potassium channels, which helps in inhibiting insulin release .
Preparation Methods
The preparation of diazoxide involves several synthetic routes. One common method includes reacting o-aminobenzenesulfonamide with N-chlorosuccinimide in a chlorine solvent to obtain 2-amino-5-chlorobenzenesulfonamide. This intermediate is then mixed with an imidazole salt and an amide solvent, followed by heating to obtain this compound . This method avoids the use of highly corrosive and toxic reagents, making it more suitable for industrial production .
Chemical Reactions Analysis
Diazoxide undergoes various chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions, although detailed pathways are less commonly discussed.
Reduction: Reduction reactions involving this compound are not widely reported.
Substitution: This compound can participate in substitution reactions, particularly involving its functional groups. Common reagents used in these reactions include N-chlorosuccinimide and imidazole salts
Scientific Research Applications
Hyperinsulinism
Diazoxide is recognized as the first-line treatment for hyperinsulinism, particularly congenital hyperinsulinism (CHI). A systematic review and meta-analysis involving six cohort studies with 1,142 participants indicated a pooled response rate of 71% (95% CI 50%-93%) for this compound therapy in managing hypoglycemic episodes due to hyperinsulinism .
Common Side Effects:
- Hypertrichosis (45%)
- Fluid retention (20%)
- Gastrointestinal reactions (13%)
- Edema (11%)
- Neutropenia (9%)
These side effects necessitate careful monitoring during treatment .
Management of Neonatal Hypoglycemia
In neonates, this compound is used to manage severe or recurrent hypoglycemia associated with conditions such as islet cell hyperplasia and extrapancreatic malignancy. A study indicated that this compound therapy could improve glycemic stability, allowing earlier weaning from intravenous fluids .
Case Study:
A documented case involved a neonate treated with this compound who exhibited significant improvement in blood glucose levels after initiating therapy. However, the treatment was also associated with fluid retention and feeding difficulties .
Efficacy and Safety
Despite its effectiveness, this compound's use is accompanied by notable adverse events. A cohort study examining children treated with this compound reported thrombocytopenia as a common adverse effect, with some patients experiencing serious complications such as multiorgan system failure . The need for well-designed randomized controlled trials has been emphasized to further validate these findings and refine treatment protocols .
Research Findings
The following table summarizes key findings from recent studies on this compound:
Mechanism of Action
Diazoxide works by activating ATP-sensitive potassium channels in the beta cells of the pancreas. This activation leads to the opening of potassium channels, causing hyperpolarization of the cell membrane. As a result, there is a decrease in calcium influx, which inhibits the release of insulin . This mechanism is the opposite of that of sulfonylureas, which increase insulin release .
Comparison with Similar Compounds
Diazoxide is chemically similar to thiazide diuretics but differs in its lack of diuretic activity. Similar compounds include:
Thiazide diuretics: Such as hydrochlorothiazide, which are used to treat hypertension and edema but have different mechanisms of action.
Sulfonylureas: Such as glibenclamide, which increase insulin release and are used to treat type 2 diabetes. This compound’s unique ability to inhibit insulin release while lacking diuretic activity sets it apart from these similar compounds.
Biological Activity
Diazoxide is a potassium channel activator primarily known for its role in managing hyperinsulinism and its neuroprotective properties. This article explores the biological activity of this compound, focusing on its mechanisms of action, therapeutic applications, and relevant clinical findings.
This compound functions by activating ATP-sensitive potassium (K-ATP) channels located in various tissues, including pancreatic beta cells and vascular smooth muscle. This activation leads to:
- Hyperpolarization of Cells : By promoting potassium efflux from cells, this compound reduces calcium influx, thereby inhibiting insulin secretion from pancreatic beta cells. This mechanism is crucial in treating conditions like hyperinsulinism, where excessive insulin secretion leads to hypoglycemia .
- Vasodilation : In vascular smooth muscle, this compound causes relaxation and dilation of arterioles, contributing to its hypotensive effects. The precise pathways involved in this vasodilation are still being elucidated but may involve antagonism of calcium channels .
1. Neuroprotection
Recent studies have highlighted this compound's neuroprotective effects, particularly in conditions associated with white matter injury, such as periventricular white matter injury (PWMI) in preterm infants. Research indicates that this compound:
- Stimulates Oligodendrocyte Proliferation : In vitro studies have shown that this compound enhances the proliferation of oligodendrocyte precursor cells (OPCs) and promotes myelination in vivo. This effect is attributed to the activation of K-ATP channels within oligodendrocytes .
- Prevents Hypoxia-Induced Injury : In animal models, this compound has been shown to prevent hypomyelination and ventriculomegaly following chronic hypoxia exposure, suggesting its potential in treating hypoxia-related neurological conditions .
2. Management of Hyperinsulinism
This compound is recognized as the first-line treatment for hyperinsulinism, especially in neonates. A systematic review and meta-analysis evaluated its efficacy:
- Response Rate : The pooled response rate for this compound therapy was found to be approximately 71% across multiple studies involving over 1,100 participants. However, the effectiveness varied significantly among studies .
- Side Effects : Common adverse effects include gastrointestinal symptoms such as decreased appetite and nausea. Monitoring is essential due to potential complications associated with long-term use .
Case Studies
Several case studies illustrate the clinical application of this compound:
- Case Study 1 : A premature infant diagnosed with hyperinsulinemic hypoglycemia showed dramatic improvement after starting oral this compound at a dosage of 12 mg/kg/day. The intravenous glucose administration was successfully tapered off within a week, demonstrating the drug's rapid effectiveness .
- Case Study 2 : In a cohort study involving 185,832 infants with hypoglycemia, this compound was administered to 1,066 infants (0.57%). The study reported an increase in this compound use over time and noted that it was often prescribed alongside diuretics .
Summary of Findings
The biological activity of this compound encompasses its role as a K-ATP channel activator with significant implications for both neuroprotection and the management of hyperinsulinism. Its ability to stimulate oligodendrocyte proliferation and prevent hypoxic damage positions it as a valuable therapeutic agent in neonatal care and neuroprotective strategies.
Data Tables
Q & A
Basic Research Questions
Q. What experimental models are most appropriate for studying diazoxide’s effects on mitochondrial function, and what methodological considerations are critical?
- Answer: In vitro models using isolated mitochondria or cell lines (e.g., NSC-34 motoneurons) are effective for assessing this compound’s modulation of ATP-sensitive potassium (KATP) channels and mitochondrial complexes. Key considerations include:
- Dose-response profiling : this compound’s biphasic effects on mitochondrial complex II/III activity require titration (e.g., 50–100 µM for KATP activation, higher doses may inhibit electron transport) .
- Temporal factors : Chronic exposure (e.g., 3 months in mice) is often necessary to observe frataxin upregulation, unlike short-term treatments .
- Combined assays : Pair electrophysiology (voltage-clamp techniques) with biochemical assays (e.g., Nrf2 nuclear translocation) to capture mechanistic interplay .
Q. How do pharmacokinetic properties of this compound influence experimental design in preclinical studies?
- Answer: this compound’s half-life (~21 hours in rodents) and metabolism (oxidation/sulfate conjugation) necessitate:
- Dosing schedules : Once-daily oral administration to maintain stable plasma levels, avoiding troughs that may confound frataxin expression or neuroprotection .
- Bioavailability monitoring : Cerebrospinal fluid (CSF) analysis in rodents shows plateau concentrations at 4 hours post-administration, informing timing of endpoint measurements .
- Species-specific adjustments : Mice require higher mg/kg doses than humans due to faster metabolic clearance .
Q. What are the primary safety concerns in this compound research, and how can they be mitigated in laboratory settings?
- Answer: this compound’s GHS hazards include acute toxicity (oral LD50: 336 mg/kg in rats) and reproductive risks (Category 1B). Mitigation strategies:
- Containment protocols : Use fume hoods for powder handling; avoid skin contact using nitrile gloves .
- In vivo safeguards**: Monitor fluid retention (common in neonates) via daily weight checks and adjust hydration protocols .
- Teratogenicity controls : Exclude pregnant animals from studies unless explicitly testing developmental toxicity, given placental transfer and beta-cell degeneration risks .
Advanced Research Questions
Q. How can researchers reconcile contradictory data on this compound’s efficacy in upregulating frataxin across models?
- Answer: Discrepancies between lymphoblastoid cells (no frataxin increase) and murine cerebellum (significant upregulation) may stem from:
- Tissue-specific KATP channel isoforms : Neuronal SUR1/Kir6.2 vs. pancreatic β-cell SUR1 variants .
- Duration and dosage : Chronic exposure (3 months) in mice vs. acute treatment (5 days) in cell cultures .
- Methodological adjustments : Use organotypic slice cultures (e.g., hippocampal or cerebellar) to bridge in vitro and in vivo findings, preserving native tissue interactions .
Q. What mechanisms underlie this compound’s neuroprotective effects beyond KATP channel activation?
- Answer: Emerging evidence highlights:
- Nrf2 pathway activation : this compound increases Nrf2 expression in NSC-34 neurons and spinal cords of EAE mice, enhancing antioxidant response elements (ARE)-driven cytoprotection .
- Mitochondrial uncoupling : At high doses, this compound disrupts supercomplex II/III interactions, reducing ROS generation but impairing electron transport .
- Anti-inflammatory modulation : Reduces pro-inflammatory cytokines (e.g., TNF-α) in demyelination models, independent of KATP channels .
Q. How should clinical trial designs for this compound address variability in hypoglycemia resolution and hyperglycemia risks?
- Answer: Lessons from neonatal hypoglycemia trials (n=74) suggest:
- Endpoint refinement : Define resolution as “enteral feeding without IV fluids for 24h + normoglycemia” to capture sustained efficacy .
- Glucose monitoring : Continuous glucose sensors (CGM) reduce testing frequency (ACR: 0.63 vs. standard) and detect hyperglycemia early .
- Stratified dosing : Weight-based titration (3–8 mg/kg/day) with renal adjustment (50% dose for CrCl <30 mL/min) to balance efficacy/safety .
Q. What experimental approaches can elucidate this compound’s dual role in glucose metabolism (hyperglycemia vs. hypoglycemia management)?
- Answer:
- Islet cell studies : Contrast this compound’s inhibition of insulin secretion (via β-cell KATP activation) with hepatic glucose output modulation .
- Genetic models : Use SUR1 knockout mice to isolate KATP-independent pathways (e.g., Nrf2) in glucose homeostasis .
- Metabolomics : Profile urine/serum metabolites (e.g., β-hydroxybutyrate) to identify thresholds for ketoacidosis risk .
Q. Data Analysis and Reporting
Q. How should researchers address high variability in this compound-induced frataxin expression in tissue samples?
- Answer:
- Normalization : Express frataxin levels relative to mitochondrial markers (e.g., VDAC1) to control for extraction efficiency .
- Statistical power : Increase sample sizes (n≥10/group) and use mixed-effects models to account for inter-individual variability .
- Cohort stratification : Group by disease stage (e.g., early vs. late Friedreich’s ataxia) to identify responder subpopulations .
Q. What biomarkers are most reliable for assessing this compound’s antioxidative effects in neurodegenerative models?
- Answer:
- Direct markers : Nrf2 nuclear translocation (immunofluorescence) and ARE-driven luciferase activity in reporter cells .
- Oxidative stress : Lipid peroxidation (MDA assay) and SOD2 activity in mitochondrial extracts .
- Functional outcomes : Myelin sheath integrity (Luxol Fast Blue staining) in cerebellar slice cultures .
Properties
IUPAC Name |
7-chloro-3-methyl-4H-1λ6,2,4-benzothiadiazine 1,1-dioxide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C8H7ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-4H,1H3,(H,10,11) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GDLBFKVLRPITMI-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NS(=O)(=O)C2=C(N1)C=CC(=C2)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C8H7ClN2O2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7022914 | |
| Record name | Diazoxide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022914 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
230.67 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
5.52e-01 g/L | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Diazoxide inhibits insulin release from the pancreas, by opening potassium channels in the beta cell membrane. Diazoxide is chemically related to thiazide diuretics but does not inhibit carbonic anhydrase and does not have chloriuretic or natriuretic activity. It also exhibits hypotensive activity by reducing arteriolar smooth muscle and vascular resistance. | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
364-98-7 | |
| Record name | Diazoxide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=364-98-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diazoxide [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000364987 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759574 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=76130 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | diazoxide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=64198 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Diazoxide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022914 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Diazoxide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.006.063 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DIAZOXIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/O5CB12L4FN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
330.5 °C | |
| Record name | Diazoxide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01119 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Diazoxide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015251 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.
















