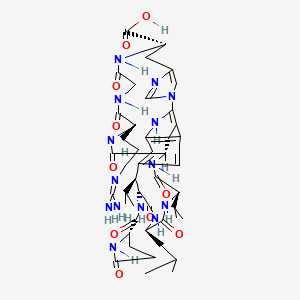
Moroidin
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Overview
Description
(8R,9S,12S,15S,18S,21S,27S)-21-[3-(diaminomethylideneamino)propyl]-12-(2-methylpropyl)-10,13,16,19,22,25-hexaoxo-9-[[(2S)-5-oxopyrrolidine-2-carbonyl]amino]-8,15-di(propan-2-yl)-2,11,14,17,20,23,26,30,32-nonazapentacyclo[16.14.2.13,7.129,32.04,33]hexatriaconta-1(33),3,5,7(36),29(35),30-hexaene-27-carboxylic acid is a natural product found in Dendrocnide moroides and Celosia argentea with data available.
Scientific Research Applications
Chemical Structure and Mechanism of Action
Moroidin consists of eight amino acids with unique structural features, including tryptophan side-chain cross-links. Its primary mechanism involves the inhibition of tubulin polymerization, which is crucial for cell division. By binding to tubulin, this compound disrupts the cytoskeletal structure of cancer cells, leading to cell cycle arrest and apoptosis. This mechanism is similar to that of established chemotherapeutic agents like vincristine and paclitaxel .
Cancer Treatment
This compound and its analogs, such as celogentin C, have shown promise as anti-cancer agents. Research indicates that these compounds exhibit cytotoxic effects against various cancer cell lines, including lung adenocarcinoma .
- Case Study: Lung Cancer
Pain Management
Interestingly, this compound's pain-inducing properties may also be leveraged for therapeutic purposes. Researchers propose that compounds causing pain can sometimes be used to modulate pain pathways effectively. By altering this compound's structure, it may be possible to develop a pain reliever that exhausts pain receptors or acts as an antagonist .
Inhibition of Vasculogenic Mimicry
This compound has been identified as a potential agent against vasculogenic mimicry (VM), a process utilized by glioblastoma cells to promote tumor growth and metastasis. Studies indicate that this compound inhibits VM formation in glioblastoma cells by affecting key signaling pathways (ERK/β-catenin) involved in epithelial-mesenchymal transition (EMT) .
Biosynthesis and Production Challenges
The natural extraction of this compound from Dendrocnide moroides poses significant challenges due to low yields. Recent advancements in biosynthesis have enabled researchers to produce this compound analogs in transgenic plants like tobacco and Nicotiana benthamiana. This method allows for scalable production while facilitating further research into this compound's pharmacological properties .
| Biosynthesis Method | Source Plant | Yield | Notes |
|---|---|---|---|
| Transgenic Expression | Nicotiana benthamiana | Increased yield | Enables large-scale production of this compound analogs |
| Natural Extraction | Dendrocnide moroides | Low yield (0.002% w/w) | Limited by availability and extraction difficulties |
Q & A
Basic Research Questions
Q. What are the primary molecular mechanisms through which Moroidin exerts its anti-cancer effects in glioblastoma models?
this compound inhibits glioblastoma (GBM) progression via dual mechanisms: (1) microtubule disruption by binding to tubulin, preventing polymerization (observed in celogentin C analogs) , and (2) suppression of epithelial-mesenchymal transition (EMT) by downregulating N-cadherin, vimentin, and β-catenin signaling. RNA sequencing and GSEA analysis revealed significant downregulation of EMT-related genes (e.g., MMP9, MMP14) and inhibition of ERK/β-catenin pathways in U87/U251 cells treated with 2 µM this compound . Western blot and immunofluorescence validated dose-dependent reduction in phosphorylated ERK and nuclear β-catenin .
Q. Which experimental assays are commonly employed to evaluate this compound's inhibitory effects on glioblastoma cell migration and vasculogenic mimicry (VM)?
Key assays include:
- Wound scratch assay : Cells cultured to 80% confluency are scratched and treated with this compound (0.5–2 µM). Migration distance is quantified via ImageJ after 24 hours .
- Transwell migration : Cells in this compound-treated upper chambers are fixed, stained, and counted after 24 hours .
- Matrigel tube formation : VM inhibition is assessed by counting tubular networks in GBM cells seeded on Matrigel. This compound (2 µM) reduces network complexity by 55% (U87) and 34% (U251) .
- qRT-PCR/Western blot : Validates downregulation of EMT markers (e.g., MMP9, vimentin) using primers/probes listed in Table 1 of .
Advanced Research Questions
Q. How can researchers reconcile contradictory findings regarding this compound's role in pain induction versus its anti-cancer properties?
While this compound was initially linked to neuropathic pain in Dendrocnide moroides, recent studies suggest its isolated form induces less pain than crude plant extracts . This discrepancy may arise from synergistic interactions with other plant toxins (e.g., histamines) or differences in in vivo vs. in vitro models. To resolve this, comparative metabolomics of plant extracts and purified this compound, paired with in vivo nociception assays (e.g., mouse paw-licking tests), are recommended .
Q. What methodologies are recommended for optimizing heterologous production of this compound analogs in non-native plant systems?
The Weng group’s gene-guided approach includes:
- BURP domain mining : Screening 793 plant transcriptomes for this compound-like motifs to identify functional cyclization enzymes .
- Copper-dependent catalysis : Utilizing copper ions to enable cyclization of tryptophan-indole macrocycles in engineered tobacco .
- Diversification : Modifying core peptide sequences in Nicotiana benthamiana to produce analogs like celogentin C, which showed selective cytotoxicity in H1437 lung adenocarcinoma cells (IC50: 1.2 µM) .
Q. How can researchers integrate multi-omics data to elucidate this compound's dual role in microtubule targeting and EMT inhibition?
A proposed workflow:
Transcriptomics : RNA-seq of this compound-treated GBM cells to identify EMT pathway suppression .
Network analysis : STRING database integration to map protein-protein interactions between EMT markers (e.g., MMP9, β-catenin) and microtubule regulators .
Functional validation : CRISPR knockdown of β-catenin in GBM cells to test if this compound’s anti-migratory effects persist .
Q. Methodological Considerations for Data Contradiction
Q. What statistical approaches are critical for analyzing dose-dependent effects of this compound in migration assays?
- Two-way ANOVA with Dunnett’s post hoc test : Used to compare migration distances across multiple doses (0.5–2 µM) and cell lines (U87 vs. U251) .
- Dose-response modeling : IC50 calculations for VM inhibition using nonlinear regression (e.g., GraphPad Prism) .
Q. How should researchers address variability in this compound's bioactivity across different glioblastoma cell lines?
- Line-specific profiling : Compare baseline expression of β-catenin and EMT markers (e.g., U87 has higher vimentin than U251) .
- Normalization to housekeeping genes : Use GAPDH or β-actin for qRT-PCR/Western blot to control for loading differences .
Q. Emerging Research Directions
Q. Can this compound analogs be engineered to enhance blood-brain barrier (BBB) penetration for glioblastoma therapy?
- Structural modifications : Introduce lipid-soluble groups (e.g., methyl esters) to celogentin C’s macrocyclic backbone .
- In vitro BBB models : Test permeability using co-cultures of brain endothelial cells and astrocytes, measuring transendothelial electrical resistance (TEER) .
Q. What in vivo models are suitable for validating this compound's anti-VM efficacy?
- Orthotopic GBM xenografts : Intracranial implantation of luciferase-tagged U87 cells in nude mice, with this compound administered via osmotic pump (2 mg/kg/day) .
- Dynamic contrast-enhanced MRI : Monitor VM reduction by tracking gadolinium leakage in tumors .
Properties
Molecular Formula |
C47H66N14O10 |
|---|---|
Molecular Weight |
987.1 g/mol |
IUPAC Name |
(8R,9S,12S,15S,18S,21S,27S)-21-[3-(diaminomethylideneamino)propyl]-12-(2-methylpropyl)-10,13,16,19,22,25-hexaoxo-9-[[(2S)-5-oxopyrrolidine-2-carbonyl]amino]-8,15-di(propan-2-yl)-2,11,14,17,20,23,26,30,32-nonazapentacyclo[16.14.2.13,7.129,32.04,33]hexatriaconta-1(33),3,5,7(36),29(35),30-hexaene-27-carboxylic acid |
InChI |
InChI=1S/C47H66N14O10/c1-21(2)14-31-43(67)59-37(23(5)6)44(68)58-32-17-27-26-10-9-24(36(22(3)4)38(45(69)57-31)60-41(65)29-11-12-34(62)53-29)15-30(26)55-39(27)61-19-25(52-20-61)16-33(46(70)71)54-35(63)18-51-40(64)28(56-42(32)66)8-7-13-50-47(48)49/h9-10,15,19-23,28-29,31-33,36-38,55H,7-8,11-14,16-18H2,1-6H3,(H,51,64)(H,53,62)(H,54,63)(H,56,66)(H,57,69)(H,58,68)(H,59,67)(H,60,65)(H,70,71)(H4,48,49,50)/t28-,29-,31-,32-,33-,36+,37-,38-/m0/s1 |
InChI Key |
UCSHFBQCLZMAJY-QFMFBHDYSA-N |
SMILES |
CC(C)CC1C(=O)NC(C(=O)NC2CC3=C(NC4=C3C=CC(=C4)C(C(C(=O)N1)NC(=O)C5CCC(=O)N5)C(C)C)N6C=C(CC(NC(=O)CNC(=O)C(NC2=O)CCCN=C(N)N)C(=O)O)N=C6)C(C)C |
Isomeric SMILES |
CC(C)C[C@H]1C(=O)N[C@H](C(=O)N[C@H]2CC3=C(NC4=C3C=CC(=C4)[C@H]([C@@H](C(=O)N1)NC(=O)[C@@H]5CCC(=O)N5)C(C)C)N6C=C(C[C@H](NC(=O)CNC(=O)[C@@H](NC2=O)CCCN=C(N)N)C(=O)O)N=C6)C(C)C |
Canonical SMILES |
CC(C)CC1C(=O)NC(C(=O)NC2CC3=C(NC4=C3C=CC(=C4)C(C(C(=O)N1)NC(=O)C5CCC(=O)N5)C(C)C)N6C=C(CC(NC(=O)CNC(=O)C(NC2=O)CCCN=C(N)N)C(=O)O)N=C6)C(C)C |
Origin of Product |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















