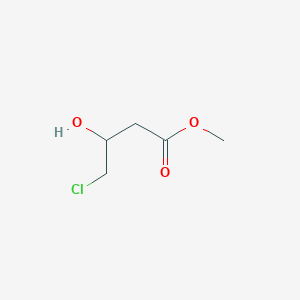
4-氯-3-羟基丁酸甲酯
描述
Methyl 4-chloro-3-hydroxybutanoate is an organic compound with the molecular formula C5H9ClO3. It is a chiral molecule, meaning it has non-superimposable mirror images, which is significant in the synthesis of enantiomerically pure compounds. This compound is often used as an intermediate in the synthesis of various pharmaceuticals, particularly those requiring chiral purity.
科学研究应用
Pharmaceutical Applications
Statin Production:
Methyl 4-chloro-3-hydroxybutanoate is recognized as a crucial intermediate in the synthesis of statins, which are widely used for cholesterol management. Statins inhibit hydroxymethylglutaryl-CoA (HMG-CoA) reductase, a key enzyme involved in cholesterol biosynthesis. The compound's ability to serve as a building block for statins underscores its significance in pharmaceutical research and development. For instance, it is involved in the synthesis of atorvastatin and simvastatin, both of which are leading medications for lowering cholesterol levels .
Chiral Intermediate:
The chiral nature of methyl 4-chloro-3-hydroxybutanoate allows it to be utilized in asymmetric synthesis processes. Asymmetric synthesis is critical for producing enantiomerically pure compounds, which are essential for the efficacy and safety of many drugs. The compound can be employed as a starting material or intermediate in the synthesis of other biologically active molecules .
Synthesis and Methodology
Various synthetic routes have been developed for producing methyl 4-chloro-3-hydroxybutanoate. These methods often involve biocatalysis or chemical transformations that enhance yield and purity:
- Biocatalytic Processes: Recent advancements have highlighted the use of biocatalysts such as keto reductases and glucose dehydrogenase in synthesizing methyl 4-chloro-3-hydroxybutanoate from suitable substrates. This method offers advantages such as mild reaction conditions and high selectivity .
- Chemical Synthesis: Traditional methods involve multi-step reactions that may include the use of epichlorohydrin and cyanide compounds under carefully controlled pH conditions to optimize yield and minimize byproducts .
Case Studies
Case Study 1: Synthesis Optimization
A study demonstrated the optimization of reaction conditions for synthesizing methyl 4-chloro-3-hydroxybutanoate using biocatalysts. The research found that adjusting pH levels between 7 to 8 significantly improved yield while maintaining optical purity, making it suitable for large-scale applications .
Case Study 2: Application in Statin Development
In another investigation, methyl 4-chloro-3-hydroxybutanoate was successfully utilized to synthesize atorvastatin with high purity. The study emphasized the compound's role as a versatile intermediate that could streamline the production process of this important drug .
作用机制
Target of Action
Methyl 4-chloro-3-hydroxybutanoate is primarily targeted by carbonyl reductase . This enzyme plays a crucial role in the biotransformation of the compound. It is often found in organisms such as Escherichia coli .
Mode of Action
The compound interacts with its target, the carbonyl reductase, through a process known as biotransformation . This interaction results in the conversion of ethyl 4-chloro-3-oxobutanoate (COBE) into ethyl (S)-4-chloro-3-hydroxybutanoate [(S)-CHBE] by the action of the carbonyl reductase .
Biochemical Pathways
The primary biochemical pathway affected by Methyl 4-chloro-3-hydroxybutanoate involves the reduction of COBE to (S)-CHBE . This process is catalyzed by the carbonyl reductase enzyme and results in the production of (S)-CHBE, a key chiral intermediate for synthesizing cholesterol-lowering drugs .
Pharmacokinetics
The compound’s bioavailability is likely influenced by its interaction with the carbonyl reductase enzyme and its subsequent biotransformation .
Result of Action
The molecular and cellular effects of Methyl 4-chloro-3-hydroxybutanoate’s action primarily involve the production of (S)-CHBE . This compound is a crucial chiral intermediate used in the synthesis of cholesterol-lowering drugs, including hydroxymethylglutaryl-CoA reductase inhibitors .
Action Environment
The action, efficacy, and stability of Methyl 4-chloro-3-hydroxybutanoate can be influenced by various environmental factors. For instance, the biotransformation of COBE into (S)-CHBE was found to be more effective when choline chloride/glycerol was used as a biocompatible solvent . Furthermore, the addition of L-glutamine and Tween-80 into the reaction media was shown to improve the biosynthesis of (S)-CHBE .
准备方法
Synthetic Routes and Reaction Conditions: Methyl 4-chloro-3-hydroxybutanoate can be synthesized through the asymmetric reduction of methyl 4-chloro-3-oxobutanoate. This reduction is typically catalyzed by stereoselective enzymes such as carbonyl reductases. The reaction conditions often involve mild temperatures and the presence of a cofactor regeneration system to maintain the activity of the enzyme .
Industrial Production Methods: In industrial settings, the production of methyl 4-chloro-3-hydroxybutanoate often employs biocatalysis due to its high enantioselectivity and efficiency. Recombinant Escherichia coli expressing carbonyl reductase and glucose dehydrogenase genes are commonly used. The process involves culturing the recombinant E. coli in large fermentors, followed by the biocatalytic reduction of methyl 4-chloro-3-oxobutanoate in an aqueous-organic solvent system .
化学反应分析
Types of Reactions: Methyl 4-chloro-3-hydroxybutanoate undergoes various chemical reactions, including:
Oxidation: It can be oxidized to form methyl 4-chloro-3-oxobutanoate.
Reduction: The compound can be reduced to form methyl 4-chloro-3-hydroxybutanoate.
Substitution: The chlorine atom can be substituted with other nucleophiles under appropriate conditions.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include potassium permanganate and chromium trioxide.
Reduction: Enzymatic reduction using carbonyl reductases is common.
Substitution: Nucleophiles such as sodium azide or thiols can be used for substitution reactions.
Major Products:
Oxidation: Methyl 4-chloro-3-oxobutanoate.
Reduction: Methyl 4-chloro-3-hydroxybutanoate.
Substitution: Various substituted derivatives depending on the nucleophile used.
相似化合物的比较
Ethyl 4-chloro-3-hydroxybutanoate: Similar in structure but with an ethyl ester group instead of a methyl ester group.
Methyl 3-hydroxybutanoate: Lacks the chlorine atom, making it less reactive in certain substitution reactions.
Methyl 4-chloro-3-oxobutanoate: The oxidized form of methyl 4-chloro-3-hydroxybutanoate.
Uniqueness: Methyl 4-chloro-3-hydroxybutanoate is unique due to its chiral nature and the presence of both a hydroxyl and a chlorine group. This combination allows for a wide range of chemical reactions and makes it a valuable intermediate in the synthesis of enantiomerically pure pharmaceuticals .
生物活性
Methyl 4-chloro-3-hydroxybutanoate (MCHB) is a chiral compound with significant biological activity, particularly in the context of enzymatic reactions and pharmaceutical applications. This article explores its biological mechanisms, biochemical properties, and relevant case studies.
Overview of Methyl 4-Chloro-3-Hydroxybutanoate
MCHB is characterized by its chiral nature, which plays a crucial role in its biological efficacy. The compound is primarily synthesized through the asymmetric reduction of ethyl 4-chloro-3-oxobutanoate, often utilizing biocatalytic methods to enhance yield and purity .
Target Enzymes
MCHB primarily interacts with the enzyme carbonyl reductase, which catalyzes the reduction of carbonyl compounds to alcohols. This interaction is vital for the synthesis of various bioactive molecules, including ethyl (R)-2-hydroxy-4-phenylbutyrate, which has therapeutic implications.
Biochemical Pathways
The action of MCHB influences several metabolic pathways. It has been shown to interact with enzymes such as alcohol dehydrogenase from Bartonella apis, affecting cellular metabolism and signaling pathways. This interaction can lead to changes in gene expression and cellular function.
MCHB exhibits several notable biochemical properties:
- Stereochemistry : The (R)-enantiomer of MCHB is often preferred in pharmaceutical applications due to its higher efficacy and selectivity compared to its (S)-enantiomer.
- Solubility : MCHB is soluble in organic solvents, which facilitates its use in various chemical reactions.
- Reactivity : The presence of the hydroxyl and chloro groups enhances its reactivity, making it a valuable intermediate in organic synthesis.
Case Studies and Research Findings
-
Enzymatic Reduction Studies
A study demonstrated that whole cells of Kluyveromyces marxianus were able to bioreduce β-ketoesters, including MCHB, effectively. The conversion rates and enantiomeric excess were analyzed using gas chromatography and infrared spectroscopy . -
Pharmaceutical Applications
MCHB serves as an intermediate in the synthesis of atorvastatin, a widely used cholesterol-lowering drug. Its role in the production process highlights its significance in medicinal chemistry . -
Toxicological Assessments
Research has indicated that MCHB exhibits low toxicity profiles in vitro. These findings are essential for evaluating its safety for use in pharmaceuticals and other applications .
Comparative Analysis
| Compound Name | Stereochemistry | Biological Activity | Use Case |
|---|---|---|---|
| Methyl 4-Chloro-3-Hydroxybutanoate | (R) | Enzyme inhibitor | Intermediate in atorvastatin synthesis |
| Ethyl 4-Chloro-3-Hydroxybutanoate | (S) | Lower efficacy | Less preferred for pharmaceutical applications |
| Ethyl (R)-2-Hydroxy-4-Phenylbutyrate | (R) | Therapeutic agent | Used in treatments for various metabolic disorders |
属性
IUPAC Name |
methyl 4-chloro-3-hydroxybutanoate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C5H9ClO3/c1-9-5(8)2-4(7)3-6/h4,7H,2-3H2,1H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WMRINGSAVOPXTE-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC(=O)CC(CCl)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C5H9ClO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID30394469 | |
| Record name | methyl 4-chloro-3-hydroxybutanoate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30394469 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
152.57 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
10488-68-3 | |
| Record name | methyl 4-chloro-3-hydroxybutanoate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID30394469 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the significance of the reaction between methyl 4-chloro-3-hydroxybutanoate and halohydrin dehalogenase?
A1: The reaction between methyl 4-chloro-3-hydroxybutanoate ((R,S)-2) and a mutated halohydrin dehalogenase (Trp249Phe) is significant because it enables the sequential kinetic resolution of the racemic mixture. [] This means the enzyme selectively converts one enantiomer of the substrate ((R)-2) into a different product, leaving the other enantiomer ((S)-2) largely unreacted. This process is highly valuable for obtaining enantiopure compounds, which are important building blocks for pharmaceuticals and other fine chemicals. In this specific case, the reaction yields (S)-methyl 4-cyano-3-hydroxybutanoate ((S)-4) with high enantiomeric excess (96.8% ee), which is a useful building block for statin side-chains. []
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。















