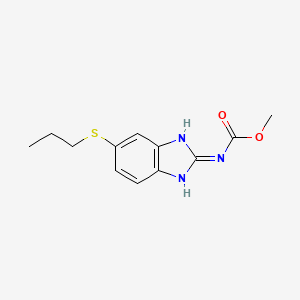
阿苯达唑
概述
描述
Albendazole is a broad-spectrum anthelmintic and antiprotozoal agent belonging to the benzimidazole class of compounds. It is primarily used to treat a variety of parasitic worm infestations, including those caused by roundworms, tapeworms, and flukes . Albendazole was developed in 1975 and is included in the World Health Organization’s List of Essential Medicines .
作用机制
阿苯达唑通过与微管的秋水仙碱敏感位点结合而发挥作用,抑制其聚合成微管。 这种微管形成的破坏会导致寄生虫肠道细胞的退行性改变,最终导致其死亡 . 主要分子靶标是微管,所涉及的途径是抑制微管聚合 .
科学研究应用
Albendazole is a benzimidazole carbamate drug with a history of safe use in humans and animals to eliminate parasitic worms . Research has shown the drug to have anti-cancer properties, and is being investigated for other applications .
Anti-Helminthic Applications
Albendazole is an anthelmintic drug, meaning it is effective against a broad spectrum of worms . Albendazole's anti-helminthic ability is attributed to its tubulin-binding capacity, which results in the de-polymerization and cell-cycle arrest in nematodes . Studies on the efficacy and safety of albendazole in humans show that it is safe and easy to administer for individuals and communities by medical and nonmedical personnel . This has enabled its use to improve general community health, including nutrition and development in children .
Efficacy of Albendazole Against Worms
| Worm | Overall Cure Rate |
|---|---|
| A. duodenale | 92% |
| A. lumbricoides | 95% |
| E. vermicularis | 98% |
| Hookworm | 78% |
| H. nana | 68% |
| N. americanus | 75% |
| S. stercoralis | 62% |
| Taenia spp. | 85% |
| Trichuris trichiura | 48% |
The recommended single dose of albendazole is 400mg . Albendazole cures significantly more hookworm infections than mebendazole .
Cancer Treatment
Albendazole is being investigated for its anti-cancer potential in melanoma, ovarian, colorectal, and hepatocellular cancer models . Albendazole has a potent anti-proliferative effect in head and neck squamous cell cancer (HNSCC) cell lines, especially in human papillomavirus (HPV)-negative lines . Albendazole can cause cell death and apoptosis, reduce cellular migration, induce cell-cycle arrest, and alter tubulin polymerization within in vitro HNSCC experimental models .
Albendazole as a Radiosensitizer
Albendazole triggers an arrest in the G2/M stage of the cell cycle, and different cell cycle phases are associated with sensitivity to radiotherapy . Studies with small-cell lung cancer and melanoma cells have demonstrated that albendazole and radiation can act in synergy to inhibit cancer cell growth . These findings suggest that albendazole may be a radiosensitizer in HNSCC treatment .
Ocular Drug Delivery
Microneedle technology uses albendazole for ocular drug delivery by addressing challenges in treating ocular diseases . Microneedles provide an advantage over intravitreal injections in localized drug delivery for treating posterior segment diseases . Dissolving microneedles produce drug micro-depots in the targeted ocular tissue’s eye layers, allowing for sustained drug release by polymeric components, offering advantages over conventional formulation strategies . The reduced size of microneedles may help prevent needle-phobia problems related to intravitreal injections and other procedures .
Adverse Effects and Safety
Albendazole has a remarkable safety record with hundreds of millions of patient exposures over a 20-year period . The incidence of side effects reported in the published literature is very low, with only gastrointestinal side effects occurring with an overall frequency of just over 1% . However, there have been some documented adverse side effects.
Case study of liver failure: A 21-year-old female developed acute hepatic failure secondary to the use of albendazole . She presented with nausea and vomiting, accompanied by malaise, mucocutaneous jaundice, dark urine, and fecal acholia .
Case study of overdose: Prolonged use of high-dose albendazole resulted in pancytopenia, hepatitis, and alopecia .
生化分析
Biochemical Properties
Albendazole interacts with tubulin, a protein that forms the cytoskeleton of cells . By binding to the colchicine-sensitive site of tubulin, Albendazole inhibits its polymerization or assembly into microtubules . This interaction disrupts the cytoskeleton of the cells, leading to their immobilization and death .
Cellular Effects
Albendazole has a significant impact on various types of cells and cellular processes. It influences cell function by disrupting the cytoskeleton, which is crucial for cell shape, division, and intracellular transport . This disruption can affect cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
The principal mode of action for Albendazole is its inhibitory effect on tubulin polymerization, which results in the loss of cytoplasmic microtubules . This mechanism leads to degenerative alterations in the tegument and intestinal cells of the worm, diminishing its energy production and leading to the immobilization and death of the parasite .
Metabolic Pathways
Albendazole is involved in metabolic pathways that lead to its transformation into metabolites: albendazole sulphoxide (ABZSO) and albendazole sulphone (ABZSO2) . These metabolites are formed in the body after administration of Albendazole .
准备方法
合成路线和反应条件
阿苯达唑可以通过多种路线合成。一种常见的方法是使用2-硝基-5-氯苯胺作为起始原料。 该化合物经历一系列反应,包括取代、缩合、还原和环化,最终得到阿苯达唑 . 该工艺避免了使用高风险的氢化还原,而是采用了碱性硫化物还原工艺,更安全且成本效益更高 .
工业生产方法
在工业环境中,阿苯达唑通常使用混合溶剂体系(水和乙醇)和混合酸体系(甲酸和乙酸)进行精制。 这种方法可以得到纯度更高、溶解度更好的阿苯达唑 . 此外,阿苯达唑可以制成结肠靶向微胶囊,以提高其吸收和疗效 .
化学反应分析
相似化合物的比较
阿苯达唑通常与其他苯并咪唑类驱虫药(如甲苯达唑和芬苯达唑)进行比较。 虽然这三种化合物具有相似的作用机制,但阿苯达唑在更广谱的活性方面具有独特性,并且对某些寄生虫感染的疗效更高 .
类似化合物
甲苯达唑: 用于治疗各种寄生虫感染,但其谱比阿苯达唑窄.
芬苯达唑: 主要用于兽药,其疗效与阿苯达唑相似.
生物活性
Albendazole (ABZ) is a broad-spectrum anthelmintic agent widely used in the treatment of various parasitic infections, including those caused by nematodes and cestodes. Its biological activity is primarily attributed to its metabolite, albendazole sulfoxide (ABZ-SO), which exhibits enhanced pharmacological effects. This article explores the biological activity of albendazole, focusing on its pharmacokinetics, mechanisms of action, clinical efficacy, and implications for treatment.
Pharmacokinetics of Albendazole
Albendazole has a complex pharmacokinetic profile characterized by high inter-individual variability in absorption and metabolism. Key pharmacokinetic parameters include:
- Absorption : Albendazole's solubility is limited in the gastrointestinal tract, which affects its bioavailability. The presence of a fatty meal significantly enhances its absorption.
- Metabolism : After oral administration, albendazole undergoes extensive first-pass metabolism in the liver, converting it to ABZ-SO. This metabolite is responsible for most of the drug's therapeutic effects.
- Half-Life : The half-life of ABZ-SO varies widely among individuals, influencing treatment outcomes.
Table 1: Key Pharmacokinetic Parameters of Albendazole
| Parameter | Value |
|---|---|
| Systemic Availability | Low (varies with food intake) |
| Half-Life of ABZ-SO | Variable (up to 8 hours) |
| Peak Concentration (CMax) | Varies with dosage |
| Area Under Curve (AUC) | Reflects total exposure |
Albendazole exerts its anthelmintic effects through several mechanisms:
- Inhibition of Microtubule Polymerization : ABZ binds to β-tubulin, disrupting microtubule formation essential for cellular processes in helminths.
- Energy Depletion : By impairing glucose uptake and ATP production in parasites, albendazole leads to energy depletion and eventual death of the organism.
- Immune Modulation : Some studies suggest that albendazole may enhance host immune responses against parasitic infections.
Clinical Efficacy and Case Studies
Albendazole has been shown to be effective against a range of parasitic infections. Notably, it is used in mass drug administration programs for soil-transmitted helminths.
Case Study 1: Hookworm Infection
A study assessed the impact of single-dose albendazole (400 mg) on hookworm-infected individuals. Results indicated significant variations in microbiota composition post-treatment:
- Individuals cured showed reduced gut microbiome diversity compared to pre-treatment.
- Those who did not clear the infection exhibited no significant changes in microbiota composition.
Table 2: Study Results on Hookworm Treatment
| Outcome | Cured Group (n=50) | Non-Cured Group (n=30) |
|---|---|---|
| Microbiota Diversity | Decreased (p=0.02) | No change (p=0.35) |
| Abundance of Clostridia | Increased | No significant change |
Case Study 2: Neurocysticercosis
In patients with neurocysticercosis, higher plasma levels of ABZ-SO correlated with improved treatment outcomes. A meta-analysis indicated that increased dosages and triple-dose regimens enhanced efficacy against this condition.
Research Findings and Implications
Recent research highlights the importance of understanding the pharmacokinetics and biological activity of albendazole to optimize treatment protocols:
- Pharmacokinetic Variability : Factors such as gastric pH and intestinal metabolism significantly influence drug absorption and efficacy.
- Microbiome Interactions : The gut microbiome's composition may predict treatment outcomes, suggesting that personalized approaches could enhance therapeutic success.
Table 3: Summary of Research Findings on Albendazole
| Study Focus | Key Findings |
|---|---|
| Pharmacokinetics | High variability; influenced by food intake |
| Mechanism of Action | Disrupts microtubules; depletes energy |
| Clinical Outcomes | Correlation between ABZ-SO levels and efficacy |
属性
IUPAC Name |
methyl N-(6-propylsulfanyl-1H-benzimidazol-2-yl)carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C12H15N3O2S/c1-3-6-18-8-4-5-9-10(7-8)14-11(13-9)15-12(16)17-2/h4-5,7H,3,6H2,1-2H3,(H2,13,14,15,16) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HXHWSAZORRCQMX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCSC1=CC2=C(C=C1)N=C(N2)NC(=O)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C12H15N3O2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0022563 | |
| Record name | Albendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022563 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
265.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Albendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014659 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1.4 [ug/mL] (The mean of the results at pH 7.4), Practically insoluble, 2.28e-02 g/L | |
| Record name | SID855809 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Albendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00518 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Albendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014659 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Albendazole causes degenerative alterations in the tegument and intestinal cells of the worm by diminishing its energy production, ultimately leading to immobilization and death of the parasite. It works by binding to the colchicine-sensitive site of tubulin, thus inhibiting its polymerization or assembly into microtubules. As cytoplasmic microtubules are critical in promoting glucose uptake in larval and adult stages of the susceptible parasites, the glycogen stores of the parasites are depleted. Degenerative changes in the endoplasmic reticulum, the mitochondria of the germinal layer, and the subsequent release of lysosomes result in decreased production of adenosine triphosphate (ATP), which is the energy required for the survival of the helminth., Benzimidazoles produce many biochemical changes in susceptible nematodes, eg, inhibition of mitochondrial fumarate reductase, reduced glucose transport, and uncoupling of oxidative phosphorylation ... /but/ the primary action ... /should be/ to inhibit microtubule polymerization by binding to beta-tubulin. The selective toxicity of these agents derives from the fact that specific, high-affinity binding to parasite beta-tubulin occurs at much lower concn than does binding to the mammalian protein ... Benzimidazole-resistant Haemonchus contortus display reduced high-affinity drug binding to beta-tubulin and alterations in beta-tubulin isotype gene expression that correlate with drug resistance ... Two identified mechanisms of drug resistance in nematodes involve both a progressive loss of "susceptible" beta-tubulin gene isotypes together with emergence of a "resistant" isotype with a conserved point mutation that encodes a tyrosine instead of phenylalanine at position 200 of beta-tubulin. While this mutation may not be required for benzimidazole resistance in all parasites, eg, Giardia lamblia, benzimidazole resistance in parasitic nematodes is unlikely to be overcome by novel benzimidazole analogs, because tyrosine also is present at position 200 of human beta-tubulin. /Benzimidazoles/, Although the exact mechanism of action of albendazole has not been fully elucidated, the principal anthelmintic effect of benzimidazoles, including albendazole, appears to be the specific, high-affinity binding of the drug to free beta-tubulin in parasite cells, resulting in selective inhibition of parasite microtubule polymerization, and inhibition of microtubule-dependent uptake of glucose. Benzimidazole drugs bind to the beta-tubulin of parasites at much lower concentrations than to mammalian beta-tubulin protein; the drugs do not inhibit glucose uptake in mammals, and do not appear to have any effect on blood glucose concentrations in humans, The mode of action of albendazole is by binding strongly with the tubulin in the cells of nematodes. The intestinal cells of the nematode are particularly affected, resulting in a loss of absorptive function which causes the nematodes to starve to death. | |
| Record name | Albendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00518 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ALBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7444 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Colorless crystals | |
CAS No. |
54965-21-8 | |
| Record name | Albendazole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=54965-21-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Albendazole [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0054965218 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Albendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00518 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | albendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758644 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | albendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=220008 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Albendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022563 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Albendazole | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.053.995 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ALBENDAZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/F4216019LN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ALBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7444 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Albendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014659 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
208-210, 208-210 °C, Mol wt: 281.34. Active metabolite of albendazole. MP: 226-228 °C (decomposes) /Sulfoxide/, 209 °C | |
| Record name | Albendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00518 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ALBENDAZOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7444 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Albendazole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014659 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details













Synthesis routes and methods II
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。














