帕罗西汀
概述
描述
帕罗西汀是一种主要用于治疗帕金森病和不安腿综合征的药物。它是一种非麦角类多巴胺激动剂,模拟大脑中神经递质多巴胺的活性。 帕罗西汀于 1997 年首次在美国获准用于医疗用途 .
作用机制
帕罗西汀通过刺激大脑中的多巴胺受体,特别是 D2、D3 和 D4 受体,发挥其作用。通过与这些受体结合,帕罗西汀增强多巴胺活性,这有助于缓解帕金森病和不安腿综合征的症状。 确切的作用机制尚不完全清楚,但据信涉及神经递质释放和神经元信号通路调节 .
类似化合物:
罗匹尼罗: 另一种用于治疗帕金森病和不安腿综合征的非麦角类多巴胺激动剂。
罗替戈汀: 一种多巴胺激动剂,以透皮贴剂的形式用于治疗帕金森病和不安腿综合征。
阿扑吗啡: 一种多巴胺激动剂,用于治疗帕金森病,特别是用于管理“脱”事件。
帕罗西汀的独特性: 帕罗西汀因其对 D3 受体的高亲和力而独一无二,这被认为有助于其在治疗帕金森病和不安腿综合征中的疗效。 此外,帕罗西汀已被证明具有神经保护和抗氧化作用,使其成为进一步研究神经退行性疾病的有希望的候选药物 .
科学研究应用
Treatment of Parkinson's Disease
Pramipexole is widely recognized for its efficacy in managing the symptoms of idiopathic Parkinson's disease. It acts as a selective agonist for dopamine receptors, particularly D2 and D3 subtypes, which are crucial in regulating motor control and behavior.
Efficacy Studies
- Clinical Trials : A multicenter, randomized, double-blind trial involving 335 patients demonstrated that pramipexole significantly reduced the severity of Parkinson's disease symptoms compared to placebo. The results were measured using the Unified Parkinson's Disease Rating Scale, showing significant improvements in both Activities of Daily Living and Motor Examination scores (p < 0.0001) .
- Long-term Outcomes : The CALM-PD study followed patients for up to 58 months, indicating that early initiation of pramipexole reduced the risk of treatment fluctuations and dyskinesias compared to levodopa treatment .
| Study | Sample Size | Duration | Key Findings |
|---|---|---|---|
| Moller et al. (2008) | 335 | 24 weeks | Significant reduction in PD symptoms (p < 0.0001) |
| Holloway et al. (2004) | 400 | 4 years | Reduced risk for treatment fluctuations with early pramipexole initiation |
Treatment of Restless Legs Syndrome
Pramipexole is also effective in treating primary Restless Legs Syndrome, a condition characterized by an uncontrollable urge to move the legs.
Meta-Analysis Findings
A meta-analysis including twelve randomized controlled trials with 3,286 participants found that pramipexole significantly improved symptoms associated with RLS. The International Restless Legs Syndrome Study Group Rating Scale scores showed a weighted mean difference favoring pramipexole over placebo .
| Outcome Measure | Pramipexole Group | Placebo Group | Weighted Mean Difference |
|---|---|---|---|
| IRLS Score Change | -4.64 | -0.00 | -4.64 (95% CI: -5.95 to -3.33) |
| Quality of Life Improvement | +5.39 | +0.00 | +5.39 (95% CI: +2.28 to +8.50) |
Potential Applications in Psychiatric Disorders
Emerging research suggests that pramipexole may have applications in treating psychiatric conditions such as bipolar disorder and major depressive disorder.
Mechanism of Action in Depression
Pramipexole's dopaminergic activity may help alleviate depressive symptoms by upregulating dopamine receptors in the mesolimbic system, an area associated with mood regulation . Studies indicate that patients with major depressive disorder exhibit downregulation of dopaminergic receptors, which pramipexole may counteract.
Neuroprotective Properties
Research indicates that pramipexole possesses neuroprotective properties, particularly against dopamine neuron degeneration due to ischemia or neurotoxicity from substances like methamphetamine . Animal studies have shown that pramipexole can block dopamine synthesis and release while promoting neuronal survival.
生化分析
Biochemical Properties
Pramipexole interacts with dopamine receptors, specifically the D2, D3, and D4 subtypes . By binding to these receptors, pramipexole stimulates dopamine activity in the brain, which helps alleviate symptoms of Parkinson’s disease and RLS. The compound has a high affinity for the D3 receptor, which is believed to contribute to its efficacy in treating these conditions .
Cellular Effects
Pramipexole influences various cellular processes, including cell signaling pathways, gene expression, and cellular metabolism. It has been shown to increase dopamine signaling in neurons, which helps restore normal motor function in patients with Parkinson’s disease . Additionally, pramipexole has neuroprotective effects, potentially due to its antioxidant properties and ability to stabilize mitochondria .
Molecular Mechanism
At the molecular level, pramipexole acts as an agonist for dopamine receptors, particularly the D2, D3, and D4 subtypes . By binding to these receptors, pramipexole stimulates dopamine activity in the brain, which helps alleviate symptoms of Parkinson’s disease and RLS. The compound also inhibits the release of somatostatin, a hormone that regulates the endocrine system .
Temporal Effects in Laboratory Settings
In laboratory settings, pramipexole has been shown to have stable effects over time. Studies have demonstrated that the compound maintains its efficacy in reducing symptoms of Parkinson’s disease and RLS over extended periods . Additionally, pramipexole has been found to have a long half-life, which contributes to its sustained effects .
Dosage Effects in Animal Models
In animal models, pramipexole has been shown to have dose-dependent effects. Lower doses of the compound are effective in alleviating symptoms of Parkinson’s disease and RLS, while higher doses can lead to adverse effects such as nausea, dizziness, and hallucinations . The optimal dosage of pramipexole varies depending on the specific condition being treated and the individual patient’s response to the medication .
Metabolic Pathways
Pramipexole undergoes minimal metabolism in the human body, with the majority of the compound being excreted unchanged in the urine . This limited metabolism reduces the risk of drug interactions and makes pramipexole a relatively safe option for long-term use. The primary route of elimination is through the kidneys, with approximately 90% of the drug being excreted in the urine .
Transport and Distribution
Pramipexole is extensively distributed throughout the body, with a volume of distribution of approximately 500 liters . The compound is about 15% bound to plasma proteins, which allows it to be readily available for interaction with dopamine receptors . Pramipexole is also distributed into red blood cells, with an erythrocyte-to-plasma ratio of approximately 2 .
Subcellular Localization
Pramipexole primarily localizes to the mitochondria within cells, where it exerts its neuroprotective effects by stabilizing mitochondrial function and reducing oxidative stress . This subcellular localization is crucial for the compound’s ability to protect neurons from damage and maintain normal cellular function.
准备方法
合成路线和反应条件: 帕罗西汀的合成涉及 (S)-2,6-二氨基-4,5,6,7-四氢苯并噻唑与烷基化剂的反应。该反应在没有碱的情况下进行,在溶剂中进行,从该溶剂中得到的 N-单烷基化产物作为盐选择性地析出。 然后用无机碱处理该盐,将其转化为游离的帕罗西汀碱,该碱可以进一步转化为药学上可接受的盐 .
工业生产方法: 在工业环境中,帕罗西汀采用多步法生产。从 4-氨基环己醇开始,该化合物经过酰化、氧化、α-卤化、闭环、水解、拆分、丙酰化和还原反应,得到帕罗西汀。 最终产物可以通过与盐酸的成盐反应转化为帕罗西汀盐酸盐 .
化学反应分析
反应类型: 帕罗西汀会发生各种化学反应,包括:
氧化: 涉及使用氧化剂,如三氯异氰尿酸。
还原: 利用还原剂,如硼氢化钠和硼烷。
取代: 涉及分子内官能团的取代。
常用试剂和条件:
氧化: 三氯异氰尿酸用作氧化剂。
还原: 硼氢化钠和硼烷是常见的还原剂。
取代: 各种烷基化剂用于取代反应。
相似化合物的比较
Ropinirole: Another non-ergot dopamine agonist used to treat Parkinson’s disease and restless legs syndrome.
Rotigotine: A dopamine agonist available as a transdermal patch for the treatment of Parkinson’s disease and restless legs syndrome.
Apomorphine: A dopamine agonist used for the treatment of Parkinson’s disease, particularly for managing “off” episodes.
Uniqueness of Pramipexole: Pramipexole is unique due to its high affinity for the D3 receptor, which is thought to contribute to its efficacy in treating Parkinson’s disease and restless legs syndrome. Additionally, pramipexole has been shown to have neuroprotective and antioxidant properties, making it a promising candidate for further research in neurodegenerative diseases .
生物活性
Pramipexole is a non-ergot dopamine agonist primarily used in the treatment of Parkinson's disease and restless legs syndrome. Its mechanism of action involves selective stimulation of dopamine D2 and D3 receptors, which plays a crucial role in regulating motor control and mood. This article explores the biological activity of pramipexole, focusing on its pharmacodynamics, clinical efficacy, and neuroprotective properties.
Pramipexole exhibits high specificity for the D2 subfamily of dopamine receptors, particularly the D3 receptor subtype. This selective binding leads to enhanced dopaminergic activity in the striatum, which is essential for motor function and mood regulation. In vitro studies have demonstrated that pramipexole can influence striatal neuronal transmission rates by activating these receptors .
Absorption and Distribution
- Bioavailability : Pramipexole has a bioavailability exceeding 90%, indicating efficient absorption.
- Volume of Distribution : Approximately 500 L, suggesting extensive distribution throughout body tissues.
- Protein Binding : About 15% of pramipexole is bound to plasma proteins, which may affect its pharmacokinetics .
Metabolism and Elimination
Pramipexole undergoes minimal metabolism in humans, primarily eliminated unchanged through renal pathways. It has shown no evidence of carcinogenicity or mutagenicity in animal studies .
Parkinson's Disease
Pramipexole has been extensively studied for its efficacy in treating Parkinson's disease (PD). A multicenter randomized trial involving 335 patients demonstrated significant reductions in PD symptoms compared to placebo, particularly in Activities of Daily Living (ADL) and Motor Examination scores on the Unified Parkinson's Disease Rating Scale (UPDRS) .
Table 1: Summary of Clinical Trials on Pramipexole for PD
Neuroprotective Effects
Research indicates that pramipexole may provide neuroprotective benefits beyond symptomatic relief. It has been shown to enhance the expression of dopaminergic neuron-associated genes such as DAT (Dopamine Transporter), VMAT2 (Vesicular Monoamine Transporter), and Nurr1, which are critical for dopamine synthesis and transport . In animal studies, pramipexole demonstrated protective effects against dopaminergic neuron degeneration following ischemic injury .
Case Studies
A recent meta-analysis assessed the efficacy of pramipexole in patients with Parkinson's disease accompanied by depression or anxiety. The study included 23 randomized controlled trials, revealing significant improvements in Hamilton Depression Rating Scale (HAMD) scores among those treated with pramipexole compared to controls .
Table 2: Meta-Analysis Results on Pramipexole for PD with Depression
| Outcome Measure | Experimental Group (Pramipexole) | Control Group (Placebo) | Statistical Significance |
|---|---|---|---|
| HAMD Score | Lowered by -3.24 | Higher | p < 0.00001 |
| Adverse Events | Fewer occurrences | More occurrences | Not statistically significant |
Safety Profile
While pramipexole is generally well-tolerated, some adverse effects have been noted, including nausea, insomnia, constipation, and hallucinations. The incidence of these side effects tends to be higher than that observed with placebo treatments . Importantly, pramipexole is classified as a pregnancy category C drug due to potential teratogenic effects observed in animal studies; thus, its use during pregnancy should be approached with caution .
属性
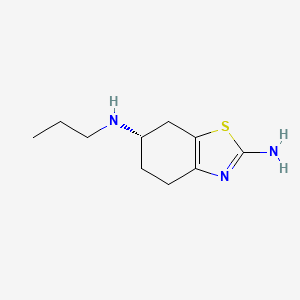
IUPAC Name |
(6S)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FASDKYOPVNHBLU-ZETCQYMHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCNC1CCC2=C(C1)SC(=N2)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCCN[C@H]1CCC2=C(C1)SC(=N2)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H17N3S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6023496 | |
| Record name | Pramipexole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6023496 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
211.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Pramipexole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014557 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
freely soluble in water, 1.40e-01 g/L | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pramipexole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014557 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The exact mechanism of action of pramipexole as a treatment for Parkinson's disease is unknown at this time. It is thought, however, that the ability of pramipexole to cause stimulation of the dopamine receptors in the striatum of the brain, a region that receives a vast array of neurological input and is responsible for a wide variety of functions, may be involved. Studies performed in animals show that pramipexole influences striatal neuronal transmission rates following activation of dopamine receptors. Pramipexole is considered a non-ergot dopamine agonist that shows specificity and strong activity at the D2 subfamily of dopamine receptors in vitro, binding selectively and dopamine D2 receptors and showing a preference for the dopamine D3 receptor subtype rather than other subtypes. The clinical significance of this binding specificity is unknown,., The purpose of this study was to determine the binding sites of pramipexole in extrastriatal dopaminergic regions because its antidepressive effects have been speculated to occur by activating the dopamine D(2) receptor subfamily in extrastriatal areas. Dynamic positron emission tomography (PET) scanning using (11)C-FLB 457 for quantification of D(2)/D(3) receptor subtype was performed on 15 healthy volunteers. Each subject underwent two PET scans before and after receiving a single dose of pramipexole (0, 0.125, or 0.25 mg). The study demonstrated that pramipexole significantly binds to D(2)/D(3) receptors in the prefrontal cortex, amygdala, and medial and lateral thalamus at a dose of 0.25 mg. These regions have been indicated to have some relation to depression and may be part of the target sites where pramipexole exerts its antidepressive effects., Pramipexole dihydrochloride, a synthetic benzothiazolamine derivative, is a nonergot-derivative dopamine receptor agonist. In in vitro binding studies, pramipexole demonstrated high binding specificity for and intrinsic activity at dopamine D2 receptors compared with other dopamine receptor agonists (e.g., bromocriptine, pergolide), having a higher affinity for the D3 receptor subtype than for the D2 or D4 subtypes. Pramipexole binds with moderate affinity to alpha2-adrenergic receptors but has little or no affinity for alpha1- or beta-adrenergic, acetylcholine, dopamine D1, or serotonin (5-hydroxytryptamine (5-HT)) receptors., Our aim was to determine if pramipexole, a D3 preferring agonist, effectively reduced dopamine neuron and fiber loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model when given at intraperitoneal doses corresponding to clinical doses. We also determined whether subchronic treatment with pramipexole regulates dopamine transporter function, thereby reducing intracellular transport of the active metabolite of MPTP, 1-methyl-4-phenylpyridinium (MPP+). Ten 12-month old C57BL/6 mice were treated with MPTP (or saline) twice per day at 20 mg/kg s.c. (4 injections over 48 h). Mice were pretreated for 3 days and during the 2-day MPTP regimen with pramipexole (0.1 mg/kg/day) or saline. Stereological quantification of dopamine neuron number and optical density measurement of dopamine fiber loss were carried out at 1 week after treatment, using immunostaining for dopamine transporter (DAT) and tyrosine hydroxylase (TH). Additional wild-type (WT) and D3 receptor knockout (KO) mice were treated for 5 days with pramipexole (0.1 mg/kg/day) or vehicle. The kinetics of (3)H-MPP+ and (3)H-DA uptake (Vmax and Km) were determined 24 hr later; and at 24 hr and 14 days dopamine transporter density was measured by quantitative autoradiography. Pramipexole treatment completely antagonized the neurotoxic effects of MPTP, as measured by substantia nigra and ventral tegmental area TH-immunoreactive cell counts. MPTP- induced loss of striatal innervation, as measured by DAT-immunoreactivity, was partially prevented by pramipexole, but not with regard to TH-IR. Pramipexole also reduced DAT- immunoreactivity in non-MPTP treated mice. Subchronic treatment with pramipexole lowered the Vmax for (3)H-DA and (3)H-MPP+ uptake into striatal synaptosomes of WT mice. Pramipexole treatment lowered Vmax in WT but not D3 KO mice; however, D3 KO mice had lower Vmax for (3)H-DA uptake. There was no change in DAT number in WT with pramipexole treatment or D3 KO mice at 24 hr post-treatment, but there was a reduction in WT-pramipexole treated and not in D3 KO mice at 14 days post-treatment. These results suggest that protection occurs at clinically suitable doses of pramipexole. Protection could be due to a reduced amount of MPP+ taken up into DA terminals via DAT. D3 receptor plays an important role in this regulation of transporter uptake and availability. | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PRAMIPEXOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8253 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
104632-26-0 | |
| Record name | Pramipexole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=104632-26-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pramipexole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0104632260 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pramipexole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6023496 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (S)-2-Amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.124.761 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PRAMIPEXOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/83619PEU5T | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PRAMIPEXOLE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8253 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Pramipexole | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014557 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
288-290 | |
| Record name | Pramipexole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00413 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。













