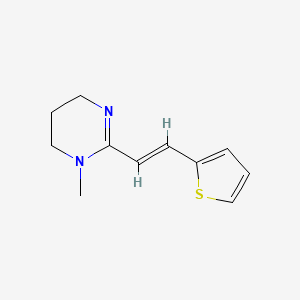
Pyrantel
Descripción general
Descripción
El pirantel es un agente antihelmíntico derivado de la pirimidina que se utiliza para tratar diversas infecciones parasitarias por gusanos, incluyendo ascaridiasis, infecciones por uncinarias, enterobiasis (infección por oxiuros), tricostrongiliasis y triquinosis . Se administra por vía oral y actúa paralizando los gusanos, que luego son expulsados del cuerpo a través de procesos naturales .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: El pirantel se sintetiza a través de una serie de reacciones químicas que involucran derivados de pirimidina. La síntesis generalmente implica la reacción de 1-metil-2-(2-tienil)vinil-5,6-dihidro-4H-pirimidina con varios reactivos bajo condiciones controladas .
Métodos de producción industrial: La producción industrial de pirantel a menudo implica el uso de cromatografía líquida de alto rendimiento (HPLC) para la purificación y análisis del compuesto. El proceso incluye el uso de acetonitrilo, ácido acético, agua y dietilamina como disolventes y reactivos .
Análisis De Reacciones Químicas
Tipos de reacciones: El pirantel experimenta diversas reacciones químicas, incluyendo reacciones de oxidación, reducción y sustitución. Estas reacciones son esenciales para modificar la estructura del compuesto y mejorar su eficacia .
Reactivos y condiciones comunes: Los reactivos comunes utilizados en las reacciones que involucran pirantel incluyen acetonitrilo, ácido acético, agua y dietilamina. Las reacciones generalmente se llevan a cabo bajo condiciones controladas de temperatura y presión para garantizar rendimientos óptimos .
Principales productos formados: Los principales productos formados a partir de las reacciones que involucran pirantel incluyen su sal de pamoato, que se utiliza en diversas formulaciones farmacéuticas .
Aplicaciones Científicas De Investigación
Clinical Applications
Pyrantel is primarily indicated for the treatment of:
- Ascariasis : Caused by Ascaris lumbricoides.
- Enterobiasis : Commonly known as pinworm infection caused by Enterobius vermicularis.
- Hookworm Infections : Particularly effective against Ancylostoma duodenale and Necator americanus.
The standard dosage for these infections is typically around 10 mg/kg for ascariasis and enterobiasis, while a 3-day treatment at the same daily dose is recommended for hookworms .
Efficacy Against Soil-Transmitted Helminths
Recent studies have demonstrated that this compound pamoate significantly reduces worm burden in cases of soil-transmitted helminths. For instance, a study showed a reduction in worm burden by 53-72% when administered post-exposure .
Post-Exposure Prophylaxis
This compound pamoate has been evaluated for its use as a post-exposure prophylactic treatment. Administering it immediately after potential exposure to larval infections can paralyze the larvae in the gastrointestinal tract, preventing them from entering systemic circulation .
Case Study 1: Efficacy in Pediatric Populations
A randomized controlled trial involving children in sub-Saharan Africa demonstrated that this compound was highly effective in treating ascariasis and enterobiasis, achieving cure rates exceeding 96% . The study highlighted its safety profile and minimal side effects, making it suitable for mass drug administration programs.
Case Study 2: Use in Veterinary Medicine
In veterinary applications, this compound tartrate has shown effectiveness against various parasitic infections in animals. Its hydrophilic nature allows systemic absorption, which is beneficial for treating infections that have entered circulation . However, this form is associated with higher toxicity risks in humans.
Conclusions
This compound remains a cornerstone in the treatment of intestinal helminth infections due to its efficacy and safety profile. Its unique mechanism of action allows it to effectively paralyze and expel parasites from the host's gastrointestinal tract. Ongoing research continues to explore its potential applications, particularly in preventive measures against parasitic infections.
Mecanismo De Acción
El pirantel actúa como un agente bloqueador neuromuscular despolarizante, causando una contracción repentina seguida de la parálisis de los helmintos. Esto da como resultado que los gusanos pierdan su agarre en la pared intestinal y sean expulsados del cuerpo . El compuesto se dirige a los receptores colinérgicos nicotínicos en los gusanos, lo que lleva a una despolarización persistente y parálisis espástica .
Compuestos similares:
- Mebendazol
- Albendazol
Comparación: El pirantel es único en su mecanismo de acción como un agente bloqueador neuromuscular despolarizante, mientras que el mebendazol y el albendazol actúan inhibiendo la polimerización de la tubulina en los gusanos . El pirantel también es conocido por su acción rápida y efectividad después de una sola dosis, lo que lo convierte en una opción preferida para tratar infecciones parasitarias por gusanos .
Comparación Con Compuestos Similares
- Mebendazole
- Albendazole
Comparison: Pyrantel is unique in its mechanism of action as a depolarizing neuromuscular blocking agent, whereas mebendazole and albendazole work by inhibiting the polymerization of tubulin in the worms . This compound is also known for its rapid action and effectiveness after a single dose, making it a preferred choice for treating parasitic worm infections .
Actividad Biológica
Pyrantel is a broad-spectrum anthelmintic agent primarily used for the treatment of various parasitic worm infections. Its biological activity is characterized by a unique mechanism of action, pharmacokinetics, and efficacy across different types of helminths, including nematodes and cestodes. This article synthesizes recent research findings, case studies, and data tables to provide a comprehensive overview of this compound's biological activity.
This compound acts as a depolarizing neuromuscular blocking agent in helminths. It promotes the release of acetylcholine and inhibits cholinesterase, leading to extensive depolarization of the muscle membrane in parasites. This action results in paralysis and detachment from the host's intestinal walls, facilitating the expulsion of the parasites through normal peristalsis . Unlike other anthelmintics such as piperazine, which induce hyperpolarization and relaxation of muscle, this compound's mechanism leads to spastic paralysis .
Pharmacokinetics
- Absorption : this compound is poorly absorbed from the gastrointestinal tract, which allows it to act locally on intestinal parasites. Peak serum concentrations occur 1–3 hours after administration .
- Volume of Distribution : The drug has a limited volume of distribution due to its poor absorption and high local concentration at the site of action.
Case Study: Efficacy Against Pinworms
A study conducted by Kobayashi et al. (2020) evaluated the effectiveness of this compound Pamoate in eliminating pinworms. The study involved administering various concentrations of this compound in a medicated gel diet to Dubia roaches and measuring fecal worm ova counts over time.
Table 1: Fecal Worm Ova Counts Post-Treatment
| Group | This compound Pamoate (mg/g) | Day 1 Total Ova | Day 9 Total Ova | Day 16 Total Ova | Day 23 Total Ova |
|---|---|---|---|---|---|
| A (Control) | 0.0 | 620 | 596 | 444 | 636 |
| B | 3.5 | 509 | 210 | 390 | 160 |
| C | 14.0 | 461 | 553 | 480 | 172 |
| D | 26.0 | 1501 | 160 | 46 | 225 |
| E | 35.0 | 510 | 176 | 358 | 194 |
The results indicated significant reductions in fecal ova counts across all treated groups compared to the control group, with Group D (26 mg/g) showing the most pronounced effects .
Post-Exposure Prophylaxis Study
Another study assessed this compound Pamoate's effectiveness as a post-exposure prophylactic treatment against Angiostrongylus cantonensis. The results showed that treatment significantly reduced worm burden by 53% to 72% when administered shortly after exposure .
Table 2: Worm Recovery Data
| Treatment Group | Mean Worm Recovery (n) |
|---|---|
| Control (No Drug) | |
| Treatment (0-h) | |
| Treatment (2-h) | |
| Treatment (4-h) |
The data indicated a significant difference between control and treatment groups, confirming this compound's efficacy when administered immediately post-infection .
Comparative Efficacy Against Other Helminths
This compound has been shown to be effective against various helminths beyond pinworms:
- Dipylidium caninum : A study demonstrated that this compound-Oxantel induced significant mortality and reduced tegument thickness in D. caninum, confirming its therapeutic potential against tapeworms .
- Caenorhabditis elegans : Early studies reported up to 98% reduction in motility in adult nematodes following treatment with this compound Pamoate, showcasing its broad-spectrum efficacy against nematodes .
Propiedades
IUPAC Name |
1-methyl-2-[(E)-2-thiophen-2-ylethenyl]-5,6-dihydro-4H-pyrimidine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C11H14N2S/c1-13-8-3-7-12-11(13)6-5-10-4-2-9-14-10/h2,4-6,9H,3,7-8H2,1H3/b6-5+ | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
YSAUAVHXTIETRK-AATRIKPKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCCN=C1C=CC2=CC=CS2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1CCCN=C1/C=C/C2=CC=CS2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C11H14N2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5023538 | |
| Record name | Pyrantel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023538 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
206.31 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
insoluble in water, TASTELESS; PRACTICALLY INSOL IN ALCOHOL OR WATER /PAMOATE/, Tastelss, yellow crystalline powder. Insol in water. /Pamoate/, Insoluble in water, slightly soluble in dimethylformamide, and soluble in dimethyl sulfoxide. | |
| Record name | Pyrantel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11156 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
By promoting the release of acetylcholine, inhibiting cholinesterase, and stimulating ganglionic neurons, pyrantel serves as a depolarizing neuromuscular blocking agent in helminths. This causes extensive depolarization of the helminth muscle membrane, resulting in tension to the helminth's muscles, leading to paralysis and release of their attachment to the host organism intestinal walls. This action is unlike piperazine, which is a hyperpolarizing neuromuscular blocking agent that causes relaxation of the helminth muscles, leading to a subsequent detachment from the intestinal wall. Excretion of the parasites in the feces occurs by normal peristalsis., PYRANTEL & ITS ANALOGS ARE DEPOLARIZING NEUROMUSCULAR BLOCKING AGENTS. THEY INDUCE MARKED, PERSISTENT ACTIVATION OF NICOTINIC RECEPTORS, WHICH RESULTS IN SPASTIC PARALYSIS OF THE WORM. PYRANTEL ALSO INHIBITS CHOLINESTERASES. /PYRANTEL PAMOATE AND ANALOGS/, PYRANTEL ... CAUSES A SLOWLY DEVELOPING CONTRACTURE OF PREPARATIONS OF ASCARIS AT 1% OF THE CONCENTRATION OF ACETYLCHOLINE REQUIRED TO PRODUCE THE SAME EFFECT. IN SINGLE MUSCLE CELLS OF THIS HELMINTH, PYRANTEL CAUSES DEPOLARIZATION & INCREASED SPIKE-DISCHARGE FREQUENCY, ACCOMPANIED BY INCREASE IN TENSION. | |
| Record name | Pyrantel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11156 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from methanol, Yellow, crystalline solid | |
CAS No. |
15686-83-6 | |
| Record name | Pyrantel | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=15686-83-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pyrantel [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015686836 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pyrantel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11156 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pyrantel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023538 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Pyrantel | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.036.143 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PYRANTEL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4QIH0N49E7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
178-179 °C, White crystals from hot methanol; max absorption (water): 312 nm (log e= 4.27); MP: 148-150 °C /Tartrate/ | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















