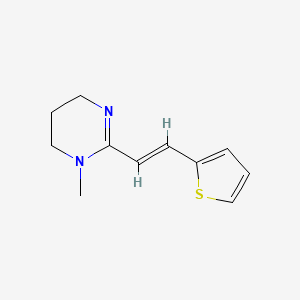
Pyrantel
概要
説明
Pyrantel is a pyrimidine-derivative anthelmintic agent used to treat various parasitic worm infections, including ascariasis, hookworm infections, enterobiasis (pinworm infection), trichostrongyliasis, and trichinellosis . It is administered orally and works by paralyzing the worms, which are then expelled from the body through natural processes .
準備方法
合成経路と反応条件: ピランテルは、ピリミジン誘導体を含む一連の化学反応によって合成されます。 合成は通常、1-メチル-2-(2-チエニル)ビニル-5,6-ジヒドロ-4H-ピリミジンとさまざまな試薬を制御された条件下で反応させることを含みます .
工業的製造方法: ピランテルの工業的製造には、多くの場合、化合物の精製と分析に高性能液体クロマトグラフィー(HPLC)が使用されます。 このプロセスには、アセトニトリル、酢酸、水、ジエチルアミンを溶媒と試薬として使用することが含まれます .
化学反応の分析
反応の種類: ピランテルは、酸化、還元、置換反応など、さまざまな化学反応を受けます。 これらの反応は、化合物の構造を変更し、有効性を高めるために不可欠です .
一般的な試薬と条件: ピランテルを含む反応で使用される一般的な試薬には、アセトニトリル、酢酸、水、ジエチルアミンなどがあります。 これらの反応は、通常、最適な収率を確保するために、制御された温度と圧力条件下で行われます .
生成される主な生成物: ピランテルを含む反応から生成される主な生成物には、さまざまな製剤で使用されているパモ酸塩が含まれます .
4. 科学研究への応用
ピランテルは、化学、生物学、医学、産業など、幅広い科学研究への応用があります。 医学では、ヒトと動物の寄生虫感染症の治療に使用されます 。 生物学では、駆虫薬が寄生虫に与える影響を研究するために使用されます 。 化学では、ピリミジン誘導体の合成と反応を研究するためのモデル化合物として使用されます 。 産業では、獣医用薬品やその他の医薬品などの製造に使用されます .
科学的研究の応用
Clinical Applications
Pyrantel is primarily indicated for the treatment of:
- Ascariasis : Caused by Ascaris lumbricoides.
- Enterobiasis : Commonly known as pinworm infection caused by Enterobius vermicularis.
- Hookworm Infections : Particularly effective against Ancylostoma duodenale and Necator americanus.
The standard dosage for these infections is typically around 10 mg/kg for ascariasis and enterobiasis, while a 3-day treatment at the same daily dose is recommended for hookworms .
Efficacy Against Soil-Transmitted Helminths
Recent studies have demonstrated that this compound pamoate significantly reduces worm burden in cases of soil-transmitted helminths. For instance, a study showed a reduction in worm burden by 53-72% when administered post-exposure .
Post-Exposure Prophylaxis
This compound pamoate has been evaluated for its use as a post-exposure prophylactic treatment. Administering it immediately after potential exposure to larval infections can paralyze the larvae in the gastrointestinal tract, preventing them from entering systemic circulation .
Case Study 1: Efficacy in Pediatric Populations
A randomized controlled trial involving children in sub-Saharan Africa demonstrated that this compound was highly effective in treating ascariasis and enterobiasis, achieving cure rates exceeding 96% . The study highlighted its safety profile and minimal side effects, making it suitable for mass drug administration programs.
Case Study 2: Use in Veterinary Medicine
In veterinary applications, this compound tartrate has shown effectiveness against various parasitic infections in animals. Its hydrophilic nature allows systemic absorption, which is beneficial for treating infections that have entered circulation . However, this form is associated with higher toxicity risks in humans.
Conclusions
This compound remains a cornerstone in the treatment of intestinal helminth infections due to its efficacy and safety profile. Its unique mechanism of action allows it to effectively paralyze and expel parasites from the host's gastrointestinal tract. Ongoing research continues to explore its potential applications, particularly in preventive measures against parasitic infections.
作用機序
ピランテルは、脱分極神経筋遮断薬として作用し、線虫の急激な収縮とそれに続く麻痺を引き起こします。 これにより、寄生虫は腸壁への付着を失い、体外に排出されます 。 この化合物は、寄生虫のニコチン性コリン作動性受容体に作用し、持続的な脱分極と痙攣性麻痺を引き起こします .
類似の化合物:
- メベンダゾール
- アルベンダゾール
比較: ピランテルは、脱分極神経筋遮断薬としての作用機序が独自であり、メベンダゾールとアルベンダゾールは寄生虫のチューブリンの重合を阻害することによって作用します 。 ピランテルは、単回投与後の急速な作用と有効性で知られており、寄生虫感染症の治療に好ましい選択肢となっています .
類似化合物との比較
- Mebendazole
- Albendazole
Comparison: Pyrantel is unique in its mechanism of action as a depolarizing neuromuscular blocking agent, whereas mebendazole and albendazole work by inhibiting the polymerization of tubulin in the worms . This compound is also known for its rapid action and effectiveness after a single dose, making it a preferred choice for treating parasitic worm infections .
生物活性
Pyrantel is a broad-spectrum anthelmintic agent primarily used for the treatment of various parasitic worm infections. Its biological activity is characterized by a unique mechanism of action, pharmacokinetics, and efficacy across different types of helminths, including nematodes and cestodes. This article synthesizes recent research findings, case studies, and data tables to provide a comprehensive overview of this compound's biological activity.
This compound acts as a depolarizing neuromuscular blocking agent in helminths. It promotes the release of acetylcholine and inhibits cholinesterase, leading to extensive depolarization of the muscle membrane in parasites. This action results in paralysis and detachment from the host's intestinal walls, facilitating the expulsion of the parasites through normal peristalsis . Unlike other anthelmintics such as piperazine, which induce hyperpolarization and relaxation of muscle, this compound's mechanism leads to spastic paralysis .
Pharmacokinetics
- Absorption : this compound is poorly absorbed from the gastrointestinal tract, which allows it to act locally on intestinal parasites. Peak serum concentrations occur 1–3 hours after administration .
- Volume of Distribution : The drug has a limited volume of distribution due to its poor absorption and high local concentration at the site of action.
Case Study: Efficacy Against Pinworms
A study conducted by Kobayashi et al. (2020) evaluated the effectiveness of this compound Pamoate in eliminating pinworms. The study involved administering various concentrations of this compound in a medicated gel diet to Dubia roaches and measuring fecal worm ova counts over time.
Table 1: Fecal Worm Ova Counts Post-Treatment
| Group | This compound Pamoate (mg/g) | Day 1 Total Ova | Day 9 Total Ova | Day 16 Total Ova | Day 23 Total Ova |
|---|---|---|---|---|---|
| A (Control) | 0.0 | 620 | 596 | 444 | 636 |
| B | 3.5 | 509 | 210 | 390 | 160 |
| C | 14.0 | 461 | 553 | 480 | 172 |
| D | 26.0 | 1501 | 160 | 46 | 225 |
| E | 35.0 | 510 | 176 | 358 | 194 |
The results indicated significant reductions in fecal ova counts across all treated groups compared to the control group, with Group D (26 mg/g) showing the most pronounced effects .
Post-Exposure Prophylaxis Study
Another study assessed this compound Pamoate's effectiveness as a post-exposure prophylactic treatment against Angiostrongylus cantonensis. The results showed that treatment significantly reduced worm burden by 53% to 72% when administered shortly after exposure .
Table 2: Worm Recovery Data
| Treatment Group | Mean Worm Recovery (n) |
|---|---|
| Control (No Drug) | |
| Treatment (0-h) | |
| Treatment (2-h) | |
| Treatment (4-h) |
The data indicated a significant difference between control and treatment groups, confirming this compound's efficacy when administered immediately post-infection .
Comparative Efficacy Against Other Helminths
This compound has been shown to be effective against various helminths beyond pinworms:
- Dipylidium caninum : A study demonstrated that this compound-Oxantel induced significant mortality and reduced tegument thickness in D. caninum, confirming its therapeutic potential against tapeworms .
- Caenorhabditis elegans : Early studies reported up to 98% reduction in motility in adult nematodes following treatment with this compound Pamoate, showcasing its broad-spectrum efficacy against nematodes .
特性
IUPAC Name |
1-methyl-2-[(E)-2-thiophen-2-ylethenyl]-5,6-dihydro-4H-pyrimidine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C11H14N2S/c1-13-8-3-7-12-11(13)6-5-10-4-2-9-14-10/h2,4-6,9H,3,7-8H2,1H3/b6-5+ | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
YSAUAVHXTIETRK-AATRIKPKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1CCCN=C1C=CC2=CC=CS2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1CCCN=C1/C=C/C2=CC=CS2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C11H14N2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID5023538 | |
| Record name | Pyrantel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023538 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
206.31 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
insoluble in water, TASTELESS; PRACTICALLY INSOL IN ALCOHOL OR WATER /PAMOATE/, Tastelss, yellow crystalline powder. Insol in water. /Pamoate/, Insoluble in water, slightly soluble in dimethylformamide, and soluble in dimethyl sulfoxide. | |
| Record name | Pyrantel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11156 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
By promoting the release of acetylcholine, inhibiting cholinesterase, and stimulating ganglionic neurons, pyrantel serves as a depolarizing neuromuscular blocking agent in helminths. This causes extensive depolarization of the helminth muscle membrane, resulting in tension to the helminth's muscles, leading to paralysis and release of their attachment to the host organism intestinal walls. This action is unlike piperazine, which is a hyperpolarizing neuromuscular blocking agent that causes relaxation of the helminth muscles, leading to a subsequent detachment from the intestinal wall. Excretion of the parasites in the feces occurs by normal peristalsis., PYRANTEL & ITS ANALOGS ARE DEPOLARIZING NEUROMUSCULAR BLOCKING AGENTS. THEY INDUCE MARKED, PERSISTENT ACTIVATION OF NICOTINIC RECEPTORS, WHICH RESULTS IN SPASTIC PARALYSIS OF THE WORM. PYRANTEL ALSO INHIBITS CHOLINESTERASES. /PYRANTEL PAMOATE AND ANALOGS/, PYRANTEL ... CAUSES A SLOWLY DEVELOPING CONTRACTURE OF PREPARATIONS OF ASCARIS AT 1% OF THE CONCENTRATION OF ACETYLCHOLINE REQUIRED TO PRODUCE THE SAME EFFECT. IN SINGLE MUSCLE CELLS OF THIS HELMINTH, PYRANTEL CAUSES DEPOLARIZATION & INCREASED SPIKE-DISCHARGE FREQUENCY, ACCOMPANIED BY INCREASE IN TENSION. | |
| Record name | Pyrantel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11156 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from methanol, Yellow, crystalline solid | |
CAS No. |
15686-83-6 | |
| Record name | Pyrantel | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=15686-83-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Pyrantel [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0015686836 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Pyrantel | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11156 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Pyrantel | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5023538 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Pyrantel | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.036.143 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | PYRANTEL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4QIH0N49E7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
178-179 °C, White crystals from hot methanol; max absorption (water): 312 nm (log e= 4.27); MP: 148-150 °C /Tartrate/ | |
| Record name | PYRANTEL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3252 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
















