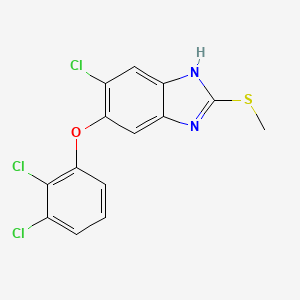
Triclabendazole
Descripción general
Descripción
Triclabendazole es un derivado de benzimidazol utilizado principalmente como agente antihelmíntico para tratar infecciones causadas por duelas hepáticas, específicamente Fasciola hepatica y Fasciola gigantica . Se comercializa bajo nombres comerciales como Egaten y Fasinex . This compound es único entre los benzimidazoles debido a su eficacia contra las etapas inmaduras y maduras de las duelas hepáticas .
Aplicaciones Científicas De Investigación
Treatment of Fascioliasis
Clinical Efficacy
Triclabendazole is the drug of choice for treating fascioliasis. Studies have demonstrated high cure rates following treatment:
- Cure Rates by Dosage :
In a retrospective cohort study involving children in Peru, the initial treatment with this compound resulted in a 55% cure rate , which decreased with subsequent treatments, indicating potential resistance or treatment failure in endemic areas .
Case Studies
- Case Study Example : In a report involving multiple cases of chronic fascioliasis, patients underwent several rounds of treatment with varying dosages of this compound. Despite initial improvements, many continued to shed Fasciola eggs post-treatment, highlighting challenges in achieving complete parasitological cure .
Potential Applications in Schistosomiasis
Recent studies have evaluated this compound's efficacy against Schistosoma mansoni , particularly in patients with co-infections of fascioliasis and schistosomiasis. A field survey showed that:
- Cure Rate for Schistosomiasis :
- In Vitro Studies : this compound demonstrated significant effects on adult schistosomes, leading to rapid destruction of their tegument within hours .
Cancer Research
Emerging research suggests that this compound may have applications in oncology:
- Mechanism of Action : It has been shown to induce pyroptosis—a form of programmed cell death—in breast cancer cells by activating caspase-3 pathways. This mechanism involves the elevation of reactive oxygen species and the regulation of apoptotic proteins .
- Tumor Volume Reduction : In xenograft models, this compound significantly reduced tumor volumes, indicating its potential as an anti-cancer agent .
Resistance and Treatment Challenges
Despite its efficacy, there are growing concerns regarding resistance to this compound:
Mecanismo De Acción
Triclabendazole y sus metabolitos son absorbidos por el tegumento de las duelas hepáticas, lo que lleva a una disminución del potencial de membrana en reposo e inhibición de la motilidad . Esta disrupción afecta la superficie y la ultraestructura de las duelas, lo que finalmente conduce a su muerte . This compound se une a la beta-tubulina, evitando la polimerización de los microtúbulos, que es esencial para la supervivencia de las duelas .
Análisis Bioquímico
Biochemical Properties
Triclabendazole and its metabolites are active against both the immature and mature worms of Fasciola hepatica and Fasciola gigantica helminths . It is mainly metabolized by the CYP1A2 enzyme into its active sulfoxide metabolite and to a lesser extent by CYP2C9, CYP2C19, CYP2D6, CYP3A, and FMO (flavin containing monooxygenase) .
Cellular Effects
This compound has been found to induce lytic cell death in MCF-7 and MDA-MB-231 breast cancer cells, a typical sign of pyroptosis . It activates apoptosis by regulating the apoptotic protein levels including Bax, Bcl-2, and enhanced cleavage of caspase-8/9/3/7 and PARP . In addition, enhanced cleavage of GSDME was also observed, which indicates the secondary necrosis/pyroptosis is further induced by active caspase-3 .
Molecular Mechanism
The molecular mode of action of this compound consists in binding to beta-tubulin, therefore preventing the polymerization of microtubules . This disrupts the structural integrity of the helminths, leading to their death .
Temporal Effects in Laboratory Settings
In a study on 350 individuals with metabolic syndrome high-risk, after a 3-month proactive intervention, two-thirds of the phenotypic markers were significantly improved in the cohort . This suggests that this compound has a time-dependent effect on biochemical markers.
Dosage Effects in Animal Models
In veterinary medicine, this compound is typically administered at an oral dose of 10 or 12 mg/kg body weight to sheep and cattle, respectively . The effects of this compound vary with different dosages in animal models. For example, in a study on sheep naturally infected with Fasciola sp., treatment with this compound resulted in significant reduction in fecal egg count .
Metabolic Pathways
This compound is metabolized within the host, principally into its sulphoxide and sulphone metabolites . This biotransformation is carried out by the flavin monooxygenase (FMO) and cytochrome P450 (CYP 450) enzyme systems .
Transport and Distribution
This compound and its metabolites are absorbed by the outer body covering of the immature and mature worms, causing a reduction in the resting membrane potential . This suggests that this compound is transported and distributed within cells and tissues via absorption.
Subcellular Localization
Given its mechanism of action, it is likely that this compound and its metabolites localize to regions where beta-tubulin is abundant, such as the cytoskeleton of cells .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
Triclabendazole se puede sintetizar utilizando varios métodos. Un método común implica comenzar con 1,2,3-triclorobenceno, que se somete a hidrólisis en licor alcalino de alta concentración para formar 2,3-diclorofenol sódico . Este intermedio reacciona con 4,5-dicloro-2-nitroanilina en una solución acuosa de metilbenceno para formar 4-cloro-5-(2,3-diclorofenoxi)-2-nitroanilina . Luego, el grupo nitro se reduce utilizando un método de transferencia catalítica de hidrógeno, y el compuesto resultante se somete a metilación para producir this compound .
Otro método implica el uso de 3,4-dicloroanilina como material de partida, seguido de acilación, nitración, hidrólisis, condensación, reducción con hidrato de hidrazina y cierre de anillo con sulfato de S-metilisotiourea . Este método evita el uso de reactivos peligrosos y reacciones de alta presión, lo que lo hace más seguro y respetuoso con el medio ambiente .
Métodos de producción industrial
La producción industrial de this compound normalmente sigue las rutas sintéticas mencionadas anteriormente, con optimizaciones para la fabricación a gran escala. El uso de materiales de partida económicos y fácilmente disponibles, junto con reactivos respetuosos con el medio ambiente, hace que el proceso sea rentable y adecuado para la producción a gran escala .
Análisis De Reacciones Químicas
Tipos de reacciones
Triclabendazole se somete a diversas reacciones químicas, que incluyen:
Oxidación: This compound se metaboliza en el hígado para formar metabolitos sulfona y sulfoxido.
Reducción: El grupo nitro en el compuesto intermedio se reduce a un grupo amino durante la síntesis.
Sustitución: La síntesis implica reacciones de sustitución nucleofílica aromática para introducir el grupo diclorofenoxi.
Reactivos y condiciones comunes
Oxidación: Las enzimas hepáticas catalizan la oxidación de this compound a sus metabolitos.
Reducción: Se utiliza transferencia catalítica de hidrógeno o hidrato de hidrazina para la reducción del grupo nitro
Sustitución: Se utiliza licor alcalino de alta concentración y solución acuosa de metilbenceno para la sustitución nucleofílica aromática.
Principales productos formados
Metabolitos sulfona y sulfoxido: Formados durante la oxidación de this compound en el hígado.
4-cloro-5-(2,3-diclorofenoxi)-2-nitroanilina: Un intermedio en la síntesis de this compound.
Comparación Con Compuestos Similares
Triclabendazole es único entre los benzimidazoles debido a su eficacia contra las duelas hepáticas inmaduras y maduras . Compuestos similares incluyen:
Albendazole: Se utiliza para tratar una variedad de infecciones parasitarias, pero es menos eficaz contra las duelas hepáticas.
Thiabendazole: Otro derivado de benzimidazol con un mecanismo de acción diferente, utilizado principalmente para tratar la strongyloidiasis.
Closantel: Efectivo contra las duelas hepáticas inmaduras, pero no tan amplio espectro como el this compound.
La estructura única de this compound, que incluye un anillo de benceno clorado y la ausencia de un grupo carbamato, contribuye a su mecanismo de acción y eficacia distintos .
Actividad Biológica
Triclabendazole (TCBZ) is a benzimidazole derivative that is primarily used as an anthelmintic agent for the treatment of infections caused by Fasciola hepatica and Fasciola gigantica. This compound exhibits a range of biological activities, particularly against helminths, and has garnered attention for its potential applications beyond parasitic infections. This article delves into the biological activity of this compound, presenting research findings, case studies, and a comprehensive analysis of its mechanisms of action.
The precise mechanism of action of this compound remains partially understood; however, several key pathways have been identified:
- Microtubule Disruption : TCBZ disrupts microtubule formation in helminths, leading to tegumental damage and impaired motility. This effect has been demonstrated in studies involving F. hepatica, where TCBZ exposure resulted in autophagic changes and loss of tubulin immunoreactivity in the tegumental syncytium .
- Metabolite Activity : The sulphoxide metabolite of TCBZ is believed to play a significant role in its efficacy. This metabolite exhibits delayed but potent effects on parasite motility and may act through multiple targets, including inhibition of adenylate cyclase activity .
- Oxidative Phosphorylation : There is evidence suggesting that TCBZ may uncouple oxidative phosphorylation, which could contribute to its anthelmintic effects .
Efficacy in Treating Fascioliasis
This compound is notably effective against all stages of Fasciola infections. A review of clinical studies indicates high cure rates following treatment with TCBZ:
| Study | Dose Regimen | Cure Rate (%) |
|---|---|---|
| Talaie et al. (2019) | 10 mg/kg (1 dose) | 63.9% |
| Talaie et al. (2019) | 10 mg/kg (2 doses) | 68.6% |
| Talaie et al. (2019) | 10 mg/kg (3 doses) | 63.9% |
| Villegas et al. (2020) | 10 mg/kg (single dose) | 70% - 100% |
Despite these high efficacy rates, treatment failures have been documented, particularly in cases with high baseline egg counts or lower socioeconomic status . A retrospective cohort study in Peru found that only 55% of children achieved parasitologic cure after the first round of treatment, with cure rates declining significantly after multiple doses .
Case Studies
Several case studies highlight the variability in treatment outcomes with this compound:
- Case Study 1 : A 51-year-old female farmer experienced significant weight loss and abdominal pain due to Fasciola infection. After initial treatment with TCBZ (10 mg/kg), her symptoms improved; however, she relapsed after eight months and continued shedding Fasciola eggs despite subsequent treatments .
- Case Study 2 : In another case involving chronic fascioliasis, a patient received multiple courses of TCBZ but failed to achieve sustained parasitologic cure, emphasizing the need for ongoing research into resistance mechanisms and alternative treatments .
Broader Biological Activities
Recent studies have also explored the antibacterial properties of this compound. Research indicates that TCBZ exhibits activity against certain Gram-positive bacteria, including methicillin-resistant strains. In combination with other agents, it has demonstrated synergistic effects against Gram-negative pathogens like E. coli and Klebsiella pneumoniae .
Propiedades
IUPAC Name |
6-chloro-5-(2,3-dichlorophenoxy)-2-methylsulfanyl-1H-benzimidazole | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H9Cl3N2OS/c1-21-14-18-9-5-8(16)12(6-10(9)19-14)20-11-4-2-3-7(15)13(11)17/h2-6H,1H3,(H,18,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NQPDXQQQCQDHHW-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CSC1=NC2=CC(=C(C=C2N1)Cl)OC3=C(C(=CC=C3)Cl)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H9Cl3N2OS | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7043952 | |
| Record name | Triclabendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7043952 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
359.7 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
0.5 [ug/mL] (The mean of the results at pH 7.4) | |
| Record name | SID50085431 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Triclabendazole is an anthelmintic agent against _Fasciola_ species. The mechanism of action against Fasciola species is not fully understood at this time. In vitro studies and animal studies suggest that triclabendazole and its active metabolites (_sulfoxide_ and _sulfone_) are absorbed by the outer body covering of the immature and mature worms, causing a reduction in the resting membrane potential, the inhibition of tubulin function as well as protein and enzyme synthesis necessary for survival. These metabolic disturbances lead to an inhibition of motility, disruption of the worm outer surface, in addition to the inhibition of spermatogenesis and egg/embryonic cells. **A note on resistance** In vitro studies, in vivo studies, as well as case reports suggest a possibility for the development of resistance to triclabendazole. The mechanism of resistance may be multifactorial and include changes in drug uptake/efflux mechanisms, target molecules, and changes in drug metabolism. The clinical significance of triclabendazole resistance in humans is not yet elucidated. | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
68786-66-3 | |
| Record name | Triclabendazole | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=68786-66-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Triclabendazole [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0068786663 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Triclabendazole | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759250 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Triclabendazole | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7043952 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1H-Benzimidazole, 6-chloro-5-(2,3-dichlorophenoxy)-2-(methylthio) | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.127.414 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TRICLABENDAZOLE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4784C8E03O | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
189-191 | |
| Record name | Triclabendazole | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB12245 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















