Dabigatran-d3
Vue d'ensemble
Description
Dabigatran-d3 est une variante marquée au deutérium du dabigatran, un anticoagulant oral de la classe des inhibiteurs directs de la thrombine. Le dabigatran est largement utilisé pour prévenir les accidents vasculaires cérébraux et la formation de caillots sanguins chez les patients atteints de fibrillation auriculaire non valvulaire et d'autres troubles thromboemboliques . Le marquage au deutérium dans this compound améliore sa stabilité et permet une quantification précise dans diverses méthodes analytiques, ce qui en fait un outil précieux dans la recherche clinique et pharmaceutique .
Applications De Recherche Scientifique
Dabigatran-d3 has a wide range of scientific research applications, including:
Chemistry: Used as a reference standard in analytical chemistry for the quantification of dabigatran in various samples.
Biology: Used in studies to understand the metabolism and pharmacokinetics of dabigatran.
Medicine: Used in clinical research to develop and validate new anticoagulant therapies.
Industry: Used in the pharmaceutical industry for quality control and regulatory compliance.
Analyse Biochimique
Biochemical Properties
Dabigatran-d3, like its parent compound dabigatran, inhibits thrombin, a key enzyme in the coagulation cascade . Thrombin plays a crucial role in blood clotting by converting fibrinogen into fibrin. By inhibiting thrombin, this compound prevents this conversion, thereby exerting its anticoagulant effect .
Cellular Effects
This compound, through its inhibition of thrombin, can impact various cellular processes. For instance, it can affect cell proliferation and adhesion, angiogenesis, and invasion, particularly in the context of cancer cells
Molecular Mechanism
This compound acts by binding to the active site of the thrombin molecule, thereby inhibiting its activity . This binding is competitive and reversible . The inhibition of thrombin prevents the conversion of fibrinogen to fibrin, impairing the clotting process and acting as an anticoagulant .
Temporal Effects in Laboratory Settings
Studies on dabigatran have shown that it has predictable anticoagulant effects, does not undergo CYP 450 metabolism, and has few drug–drug and drug–food interactions .
Dosage Effects in Animal Models
The effects of this compound at different dosages in animal models have not been extensively studied. Studies on dabigatran have shown that it has a predictable pharmacokinetic profile, with a clearance of 0.0453 L/min/70 kg, and a central volume of distribution of 2.94 L/70 kg .
Metabolic Pathways
This compound is likely to be involved in similar metabolic pathways as dabigatran. Dabigatran is metabolized by esterases and is a substrate of P-glycoprotein (P-gp) and uridine 5ʹ-diphospho (UDP)-glucuronosyltransferases (UGTs) .
Transport and Distribution
This compound, like dabigatran, is likely to be transported and distributed within cells and tissues via P-gp, a protein that pumps foreign substances out of cells
Méthodes De Préparation
Voies de synthèse et conditions de réaction : La synthèse de Dabigatran-d3 implique plusieurs étapes, à partir du bromobenzène marqué au deutérium. Le processus comprend la nitration, la cyanation, la réaction de Pinner, l'estérification, la réduction et l'alkylation . Le produit final est obtenu par condensation du dérivé O-n-hexylcarbamate marqué au deutérium avec du benzimidazole marqué au deutérium .
Méthodes de production industrielle : La production industrielle de this compound suit des voies de synthèse similaires mais à plus grande échelle. Le processus est optimisé pour un rendement et une pureté élevés, garantissant que le produit final répond aux normes de qualité strictes requises pour les applications pharmaceutiques .
Analyse Des Réactions Chimiques
Types de réactions : Dabigatran-d3 subit diverses réactions chimiques, notamment :
Réduction : Implique l'élimination d'atomes d'oxygène ou l'ajout d'atomes d'hydrogène.
Substitution : Implique le remplacement d'un atome ou d'un groupe d'atomes par un autre.
Réactifs et conditions courants :
Oxydation : Les réactifs courants comprennent le permanganate de potassium et le peroxyde d'hydrogène.
Réduction : Les réactifs courants comprennent l'hydrure de lithium et d'aluminium et le borohydrure de sodium.
Substitution : Les réactifs courants comprennent les halogènes et les nucléophiles en conditions acides ou basiques.
Principaux produits formés : Les principaux produits formés à partir de ces réactions dépendent des conditions et des réactifs spécifiques utilisés. Par exemple, l'oxydation peut produire des acides carboxyliques, tandis que la réduction peut produire des alcools .
4. Applications de la recherche scientifique
This compound a un large éventail d'applications de recherche scientifique, notamment :
Biologie : Utilisé dans des études pour comprendre le métabolisme et la pharmacocinétique du dabigatran.
Médecine : Utilisé dans la recherche clinique pour développer et valider de nouvelles thérapies anticoagulantes.
Industrie : Utilisé dans l'industrie pharmaceutique pour le contrôle qualité et la conformité réglementaire.
5. Mécanisme d'action
This compound, comme le dabigatran, est un inhibiteur direct de la thrombine. Il agit en se liant au site actif de la thrombine, une sérine protéase qui convertit le fibrinogène en fibrine pendant la cascade de la coagulation . En inhibant la thrombine, this compound empêche la formation de caillots sanguins, réduisant ainsi le risque d'accident vasculaire cérébral et d'autres événements thromboemboliques .
Composés similaires :
Apixaban : Un autre anticoagulant oral direct qui inhibe le facteur Xa.
Rivaroxaban : Un anticoagulant oral direct qui inhibe également le facteur Xa.
Edoxaban : Un anticoagulant oral direct qui inhibe le facteur Xa.
Comparaison :
Mécanisme d'action : Alors que this compound inhibe la thrombine, les autres composés inhibent le facteur Xa.
Pharmacocinétique : this compound a un profil pharmacocinétique prévisible et ne nécessite pas de surveillance fréquente, de manière similaire aux autres anticoagulants oraux directs.
Caractéristiques uniques : Le marquage au deutérium dans this compound améliore sa stabilité et permet une quantification précise, ce qui le rend unique parmi les anticoagulants.
Mécanisme D'action
Dabigatran-d3, like dabigatran, is a direct thrombin inhibitor. It works by binding to the active site of thrombin, a serine protease that converts fibrinogen to fibrin during the coagulation cascade . By inhibiting thrombin, this compound prevents the formation of blood clots, thereby reducing the risk of stroke and other thromboembolic events .
Comparaison Avec Des Composés Similaires
Apixaban: Another direct oral anticoagulant that inhibits factor Xa.
Rivaroxaban: A direct oral anticoagulant that also inhibits factor Xa.
Edoxaban: A direct oral anticoagulant that inhibits factor Xa.
Comparison:
Mechanism of Action: While Dabigatran-d3 inhibits thrombin, the other compounds inhibit factor Xa.
Pharmacokinetics: this compound has a predictable pharmacokinetic profile and does not require frequent monitoring, similar to the other direct oral anticoagulants.
Unique Features: The deuterium labeling in this compound enhances its stability and allows for precise quantification, making it unique among anticoagulants.
Activité Biologique
Dabigatran-d3 is a deuterated form of dabigatran, a direct thrombin inhibitor used primarily as an anticoagulant. Understanding the biological activity of this compound involves examining its pharmacodynamics, mechanisms of action, and clinical implications. This article synthesizes findings from various studies to provide a comprehensive overview of this compound's biological activity.
Dabigatran functions as a direct thrombin inhibitor , selectively binding to the active site of thrombin, which prevents the conversion of fibrinogen to fibrin. This inhibition is crucial in the coagulation cascade, significantly reducing thrombus formation.
- Inhibition Potency : Studies have demonstrated that dabigatran exhibits a high binding affinity for human thrombin with an inhibition constant (Ki) of approximately 4.5 nM. It also effectively inhibits thrombin-induced platelet aggregation with an IC50 value around 10 nM .
Pharmacokinetics and Pharmacodynamics
The pharmacokinetic profile of this compound is similar to that of its parent compound, dabigatran. Key pharmacokinetic parameters include:
- Absorption : Following oral administration, dabigatran etexilate (the prodrug form) is converted to dabigatran, with peak plasma concentrations typically reached within 1.5 to 2 hours.
- Elimination : The drug is primarily eliminated via renal pathways, with about 85% excreted unchanged in urine .
Table 1: Pharmacokinetic Parameters of Dabigatran
| Parameter | Value |
|---|---|
| Peak Plasma Concentration (Cmax) | ~1.25-1.5 hours post-dose |
| Elimination Half-life | ~12-17 hours |
| Bioavailability | ~6.5% (after oral dosing) |
| Renal Excretion | ~85% |
Anticoagulant Activity
This compound demonstrates significant anticoagulant effects, which can be measured through various coagulation assays:
- Activated Partial Thromboplastin Time (aPTT) : Dabigatran prolongs aPTT in a dose-dependent manner, indicating its effectiveness in inhibiting thrombin activity .
- Prothrombin Time (PT) : Similar effects are observed with PT measurements.
- Ecarin Clotting Time (ECT) : ECT is also significantly prolonged by dabigatran administration.
Table 2: Coagulation Assay Results
| Assay Type | Dose (mg/kg) | aPTT Prolongation (seconds) | PT Prolongation (seconds) |
|---|---|---|---|
| Intravenous in Rats | 0.3 | 30 | 5 |
| Intravenous in Rats | 1 | 50 | 10 |
| Oral in Monkeys | 5 | 60 | 12 |
Clinical Implications
This compound has been studied for its potential therapeutic applications beyond anticoagulation. For instance:
- Neurological Effects : Research indicates that dabigatran can suppress thrombin-induced activation in astrocytes, leading to reduced inflammation and improved neurological function in models of multiple sclerosis . This suggests a potential role for dabigatran in treating neuroinflammatory conditions.
Case Studies
A notable case study involved the evaluation of dabigatran's efficacy in trauma patients. The study utilized a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay to detect dabigatran and its metabolites with high sensitivity and specificity, confirming the drug's presence in patients undergoing anticoagulation therapy .
Propriétés
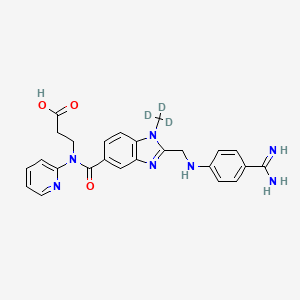
IUPAC Name |
3-[[2-[(4-carbamimidoylanilino)methyl]-1-(trideuteriomethyl)benzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34)/i1D3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
YBSJFWOBGCMAKL-FIBGUPNXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1C2=C(C=C(C=C2)C(=O)N(CCC(=O)O)C3=CC=CC=N3)N=C1CNC4=CC=C(C=C4)C(=N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])N1C2=C(C=C(C=C2)C(=O)N(CCC(=O)O)C3=CC=CC=N3)N=C1CNC4=CC=C(C=C4)C(=N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H25N7O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID70747410 | |
| Record name | N-{2-[(4-Carbamimidoylanilino)methyl]-1-(~2~H_3_)methyl-1H-benzimidazole-5-carbonyl}-N-pyridin-2-yl-beta-alanine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID70747410 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
474.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1246817-44-6 | |
| Record name | N-{2-[(4-Carbamimidoylanilino)methyl]-1-(~2~H_3_)methyl-1H-benzimidazole-5-carbonyl}-N-pyridin-2-yl-beta-alanine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID70747410 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details




Q1: What is the role of Dabigatran-d3 in quantifying dabigatran levels?
A1: this compound is a deuterated form of dabigatran used as an internal standard (IS) in mass spectrometry-based analytical methods. [, ] These methods are essential for accurately determining the concentration of free dabigatran in biological samples, such as plasma.
Q2: How does the use of this compound as an IS improve the accuracy of dabigatran quantification?
A2: Using an IS like this compound offers several advantages:
- Compensation for variations: It corrects for potential sample loss during preparation and variation in ionization efficiency during analysis. [, ]
- Improved accuracy: By comparing the signal of dabigatran with the signal of the known concentration of this compound, researchers can accurately calculate the dabigatran concentration in the sample. [, ]
- Enhanced reliability: This approach ensures the reliability and reproducibility of the measurements, which is crucial for pharmacokinetic studies and therapeutic drug monitoring of dabigatran. [, ]
Q3: Can you give an example of how this compound was used in a research setting?
A3: In a study examining the bioequivalence of generic and brand name dabigatran etexilate, researchers utilized a UPLC-MS/MS method with this compound as the IS. [] This allowed them to accurately quantify free dabigatran in patient plasma samples and compare the pharmacokinetic profiles of the two formulations. []
- Development and Validation of UPLC-MS/MS Method for Quantifying of Free and Total Dabigatran in Human Plasma: An Application for a Bioequivalence Study.
- Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.













