Dabigatran-d3
Übersicht
Beschreibung
Dabigatran-d3 is a deuterium-labeled variant of dabigatran, an oral anticoagulant from the direct thrombin inhibitor class. Dabigatran is widely used to prevent strokes and the formation of blood clots in patients with non-valvular atrial fibrillation and other thromboembolic disorders . The deuterium labeling in this compound enhances its stability and allows for precise quantification in various analytical methods, making it a valuable tool in clinical and pharmaceutical research .
Wissenschaftliche Forschungsanwendungen
Dabigatran-d3 hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung, darunter:
Biologie: Wird in Studien zur Untersuchung des Metabolismus und der Pharmakokinetik von Dabigatran verwendet.
5. Wirkmechanismus
This compound ist wie Dabigatran ein direkter Thrombininhibitor. Es wirkt, indem es an die aktive Stelle von Thrombin bindet, einer Serinprotease, die während der Gerinnungskaskade Fibrinogen in Fibrin umwandelt . Durch die Hemmung von Thrombin verhindert this compound die Bildung von Blutgerinnseln und verringert so das Risiko von Schlaganfällen und anderen thromboembolischen Ereignissen .
Ähnliche Verbindungen:
Apixaban: Ein weiteres direktes orales Antikoagulans, das Faktor Xa hemmt.
Rivaroxaban: Ein direktes orales Antikoagulans, das ebenfalls Faktor Xa hemmt.
Edoxaban: Ein direktes orales Antikoagulans, das Faktor Xa hemmt.
Vergleich:
Wirkmechanismus: Während this compound Thrombin hemmt, hemmen die anderen Verbindungen Faktor Xa.
Pharmakokinetik: this compound hat ein vorhersehbares pharmakokinetisches Profil und erfordert keine häufige Überwachung, ähnlich wie die anderen direkten oralen Antikoagulanzien.
Einzigartige Merkmale: Die Deuteriummarkierung in this compound erhöht seine Stabilität und ermöglicht die präzise Quantifizierung, was es unter den Antikoagulanzien einzigartig macht.
Zusammenfassend lässt sich sagen, dass this compound aufgrund seiner Stabilität, seiner präzisen Quantifizierungsmöglichkeiten und seiner wirksamen Antikoagulanz Eigenschaften eine wertvolle Verbindung in der wissenschaftlichen Forschung und in klinischen Anwendungen ist.
Biochemische Analyse
Biochemical Properties
Dabigatran-d3, like its parent compound dabigatran, inhibits thrombin, a key enzyme in the coagulation cascade . Thrombin plays a crucial role in blood clotting by converting fibrinogen into fibrin. By inhibiting thrombin, this compound prevents this conversion, thereby exerting its anticoagulant effect .
Cellular Effects
This compound, through its inhibition of thrombin, can impact various cellular processes. For instance, it can affect cell proliferation and adhesion, angiogenesis, and invasion, particularly in the context of cancer cells
Molecular Mechanism
This compound acts by binding to the active site of the thrombin molecule, thereby inhibiting its activity . This binding is competitive and reversible . The inhibition of thrombin prevents the conversion of fibrinogen to fibrin, impairing the clotting process and acting as an anticoagulant .
Temporal Effects in Laboratory Settings
Studies on dabigatran have shown that it has predictable anticoagulant effects, does not undergo CYP 450 metabolism, and has few drug–drug and drug–food interactions .
Dosage Effects in Animal Models
The effects of this compound at different dosages in animal models have not been extensively studied. Studies on dabigatran have shown that it has a predictable pharmacokinetic profile, with a clearance of 0.0453 L/min/70 kg, and a central volume of distribution of 2.94 L/70 kg .
Metabolic Pathways
This compound is likely to be involved in similar metabolic pathways as dabigatran. Dabigatran is metabolized by esterases and is a substrate of P-glycoprotein (P-gp) and uridine 5ʹ-diphospho (UDP)-glucuronosyltransferases (UGTs) .
Transport and Distribution
This compound, like dabigatran, is likely to be transported and distributed within cells and tissues via P-gp, a protein that pumps foreign substances out of cells
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: The synthesis of Dabigatran-d3 involves multiple steps, starting from deuterium-labeled bromobenzene. The process includes nitration, cyanation, Pinner reaction, esterification, reduction, and alkylation . The final product is obtained through the condensation of deuterium-labeled O-n-hexylcarbamate derivative with deuterium-labeled benzimidazole .
Industrial Production Methods: Industrial production of this compound follows similar synthetic routes but on a larger scale. The process is optimized for high yield and purity, ensuring that the final product meets stringent quality standards required for pharmaceutical applications .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Dabigatran-d3 durchläuft verschiedene chemische Reaktionen, darunter:
Reduktion: Umfasst die Entfernung von Sauerstoffatomen oder die Addition von Wasserstoffatomen.
Substitution: Umfasst den Austausch eines Atoms oder einer Atomgruppe gegen ein anderes.
Häufige Reagenzien und Bedingungen:
Oxidation: Häufige Reagenzien sind Kaliumpermanganat und Wasserstoffperoxid.
Reduktion: Häufige Reagenzien sind Lithiumaluminiumhydrid und Natriumborhydrid.
Substitution: Häufige Reagenzien sind Halogene und Nukleophile unter sauren oder basischen Bedingungen.
Hauptprodukte, die gebildet werden: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, hängen von den spezifischen Bedingungen und Reagenzien ab, die verwendet werden. Zum Beispiel kann die Oxidation zu Carbonsäuren führen, während die Reduktion zu Alkoholen führen kann .
Wirkmechanismus
Dabigatran-d3, like dabigatran, is a direct thrombin inhibitor. It works by binding to the active site of thrombin, a serine protease that converts fibrinogen to fibrin during the coagulation cascade . By inhibiting thrombin, this compound prevents the formation of blood clots, thereby reducing the risk of stroke and other thromboembolic events .
Vergleich Mit ähnlichen Verbindungen
Apixaban: Another direct oral anticoagulant that inhibits factor Xa.
Rivaroxaban: A direct oral anticoagulant that also inhibits factor Xa.
Edoxaban: A direct oral anticoagulant that inhibits factor Xa.
Comparison:
Mechanism of Action: While Dabigatran-d3 inhibits thrombin, the other compounds inhibit factor Xa.
Pharmacokinetics: this compound has a predictable pharmacokinetic profile and does not require frequent monitoring, similar to the other direct oral anticoagulants.
Unique Features: The deuterium labeling in this compound enhances its stability and allows for precise quantification, making it unique among anticoagulants.
Biologische Aktivität
Dabigatran-d3 is a deuterated form of dabigatran, a direct thrombin inhibitor used primarily as an anticoagulant. Understanding the biological activity of this compound involves examining its pharmacodynamics, mechanisms of action, and clinical implications. This article synthesizes findings from various studies to provide a comprehensive overview of this compound's biological activity.
Dabigatran functions as a direct thrombin inhibitor , selectively binding to the active site of thrombin, which prevents the conversion of fibrinogen to fibrin. This inhibition is crucial in the coagulation cascade, significantly reducing thrombus formation.
- Inhibition Potency : Studies have demonstrated that dabigatran exhibits a high binding affinity for human thrombin with an inhibition constant (Ki) of approximately 4.5 nM. It also effectively inhibits thrombin-induced platelet aggregation with an IC50 value around 10 nM .
Pharmacokinetics and Pharmacodynamics
The pharmacokinetic profile of this compound is similar to that of its parent compound, dabigatran. Key pharmacokinetic parameters include:
- Absorption : Following oral administration, dabigatran etexilate (the prodrug form) is converted to dabigatran, with peak plasma concentrations typically reached within 1.5 to 2 hours.
- Elimination : The drug is primarily eliminated via renal pathways, with about 85% excreted unchanged in urine .
Table 1: Pharmacokinetic Parameters of Dabigatran
| Parameter | Value |
|---|---|
| Peak Plasma Concentration (Cmax) | ~1.25-1.5 hours post-dose |
| Elimination Half-life | ~12-17 hours |
| Bioavailability | ~6.5% (after oral dosing) |
| Renal Excretion | ~85% |
Anticoagulant Activity
This compound demonstrates significant anticoagulant effects, which can be measured through various coagulation assays:
- Activated Partial Thromboplastin Time (aPTT) : Dabigatran prolongs aPTT in a dose-dependent manner, indicating its effectiveness in inhibiting thrombin activity .
- Prothrombin Time (PT) : Similar effects are observed with PT measurements.
- Ecarin Clotting Time (ECT) : ECT is also significantly prolonged by dabigatran administration.
Table 2: Coagulation Assay Results
| Assay Type | Dose (mg/kg) | aPTT Prolongation (seconds) | PT Prolongation (seconds) |
|---|---|---|---|
| Intravenous in Rats | 0.3 | 30 | 5 |
| Intravenous in Rats | 1 | 50 | 10 |
| Oral in Monkeys | 5 | 60 | 12 |
Clinical Implications
This compound has been studied for its potential therapeutic applications beyond anticoagulation. For instance:
- Neurological Effects : Research indicates that dabigatran can suppress thrombin-induced activation in astrocytes, leading to reduced inflammation and improved neurological function in models of multiple sclerosis . This suggests a potential role for dabigatran in treating neuroinflammatory conditions.
Case Studies
A notable case study involved the evaluation of dabigatran's efficacy in trauma patients. The study utilized a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay to detect dabigatran and its metabolites with high sensitivity and specificity, confirming the drug's presence in patients undergoing anticoagulation therapy .
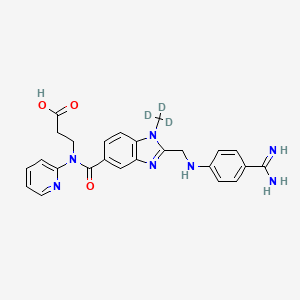
Eigenschaften
IUPAC Name |
3-[[2-[(4-carbamimidoylanilino)methyl]-1-(trideuteriomethyl)benzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34)/i1D3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
YBSJFWOBGCMAKL-FIBGUPNXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1C2=C(C=C(C=C2)C(=O)N(CCC(=O)O)C3=CC=CC=N3)N=C1CNC4=CC=C(C=C4)C(=N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])N1C2=C(C=C(C=C2)C(=O)N(CCC(=O)O)C3=CC=CC=N3)N=C1CNC4=CC=C(C=C4)C(=N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H25N7O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID70747410 | |
| Record name | N-{2-[(4-Carbamimidoylanilino)methyl]-1-(~2~H_3_)methyl-1H-benzimidazole-5-carbonyl}-N-pyridin-2-yl-beta-alanine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID70747410 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
474.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1246817-44-6 | |
| Record name | N-{2-[(4-Carbamimidoylanilino)methyl]-1-(~2~H_3_)methyl-1H-benzimidazole-5-carbonyl}-N-pyridin-2-yl-beta-alanine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID70747410 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details




Q1: What is the role of Dabigatran-d3 in quantifying dabigatran levels?
A1: this compound is a deuterated form of dabigatran used as an internal standard (IS) in mass spectrometry-based analytical methods. [, ] These methods are essential for accurately determining the concentration of free dabigatran in biological samples, such as plasma.
Q2: How does the use of this compound as an IS improve the accuracy of dabigatran quantification?
A2: Using an IS like this compound offers several advantages:
- Compensation for variations: It corrects for potential sample loss during preparation and variation in ionization efficiency during analysis. [, ]
- Improved accuracy: By comparing the signal of dabigatran with the signal of the known concentration of this compound, researchers can accurately calculate the dabigatran concentration in the sample. [, ]
- Enhanced reliability: This approach ensures the reliability and reproducibility of the measurements, which is crucial for pharmacokinetic studies and therapeutic drug monitoring of dabigatran. [, ]
Q3: Can you give an example of how this compound was used in a research setting?
A3: In a study examining the bioequivalence of generic and brand name dabigatran etexilate, researchers utilized a UPLC-MS/MS method with this compound as the IS. [] This allowed them to accurately quantify free dabigatran in patient plasma samples and compare the pharmacokinetic profiles of the two formulations. []
- Development and Validation of UPLC-MS/MS Method for Quantifying of Free and Total Dabigatran in Human Plasma: An Application for a Bioequivalence Study.
- Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.













