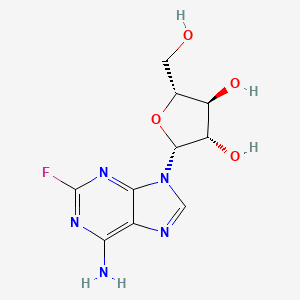
フルダラビン
概要
説明
フルダラビンはプリンアナログであり、抗悪性腫瘍剤ですこの化合物は主に、慢性リンパ性白血病、非ホジキンリンパ腫、急性骨髄性白血病、急性リンパ性白血病などの血液悪性腫瘍の治療に使用されます . フルダラビンはDNAの複製を阻害することで作用し、重要な化学療法剤となっています .
2. 製法
合成経路と反応条件: フルダラビンは、2-フルオロ-9-β-D-(2',3',5'-トリアルコキシアラビノフラノシル)アデニンを原料として合成できます。反応は、水酸化ナトリウムとアンモニア水の混合溶液を反応剤とし、水と2-メチルテトラヒドロフランの混合溶液を溶媒として行います。 反応は0-5°Cで1-3時間行い、その後、氷酢酸で中和し、減圧ろ過、再結晶、活性炭による脱色を行い、純粋なフルダラビンを得ます .
工業的製造方法: フルダラビンの工業的製造は、フルダラビンとオキシ塩化リンの酯化と加水分解を伴います。 このプロセスは、プリン環と糖環の母核を初期原料とし、その後、多段階操作と脱保護によって目的物を得ます .
3. 化学反応解析
反応の種類: フルダラビンは、以下を含むさまざまな化学反応を起こします。
酸化: フルダラビンは酸化されて異なる代謝物を形成することができます。
還元: 還元反応は、フルダラビン分子の官能基を修飾することができます。
置換: 置換反応は、プリン環または糖部分で起こります。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムがあります。
還元: 水素化ホウ素ナトリウムや水素化リチウムアルミニウムなどの還元剤が使用されます。
置換: 置換反応は、多くの場合、塩化物やアミンなどの求核剤を塩基性または酸性条件下で使用して行われます。
主な生成物: これらの反応から生成される主な生成物には、さまざまな代謝物とフルダラビンの誘導体があり、薬理学的特性が異なる場合があります .
4. 科学研究への応用
フルダラビンは、幅広い科学研究への応用があります。
化学: ヌクレオシドアナログとそのDNAとの相互作用を研究するためのモデル化合物として使用されます。
生物学: DNA合成と修復機構の研究に使用されます。
作用機序
フルダラビンリン酸は、迅速に脱リン酸化されて2-フルオロ-ara-Aとなり、その後、デオキシシチジンキナーゼによって細胞内で活性なトリリン酸、2-フルオロ-ara-ATPにリン酸化されます。 この代謝物は、DNAポリメラーゼα、リボヌクレオチドレダクターゼ、DNAプライマーゼを阻害し、DNA合成を阻害し、癌細胞を破壊します .
類似化合物:
クロファラビン: 類似のメカニズムを持つ別のプリンヌクレオシドアナログですが、初期の白血病設定では毒性がより高くなります.
ベンダムスチン: 潜在的に同様の奏効率と毒性の低下を示す、代替のリンパ球枯渇療法として使用されます.
独自性: フルダラビンは、リンパ球に対する高い選択性と、分裂期および休止期の両方の細胞におけるDNA合成を阻害する能力が特徴です。 その免疫抑制効果は、幹細胞移植前のコンディショニングレジメンでも価値があります .
科学的研究の応用
Chronic Lymphocytic Leukemia (CLL)
Fludarabine has become a standard treatment for CLL, particularly in patients with advanced disease or those who are refractory to previous therapies. The drug is often used alone or in combination with other agents like cyclophosphamide and rituximab.
Case Study: Fludarabine-Cyclophosphamide-Rituximab Regimen
- Patient Cohort : 300 patients with CLL
- Overall Response Rate : 95%
- Complete Remission : 72%
- Median Time to Progression : 80 months
- Survival Rates : Six-year overall survival was reported at 77% .
Allogeneic Stem Cell Transplantation
Fludarabine is frequently included in conditioning regimens prior to allogeneic stem cell transplantation due to its immunosuppressive effects, which facilitate engraftment and reduce the risk of graft-versus-host disease.
Research Findings:
- Fludarabine enhances the engraftment of human hematopoietic cells in immunocompromised mouse models.
- Studies indicate that fludarabine-treated mice exhibit improved levels of human hematopoiesis compared to controls .
Multiple Myeloma
In multiple myeloma, fludarabine has been studied as part of combination therapies aimed at improving response rates and prolonging survival.
Clinical Trial Results:
- A study demonstrated that the addition of fludarabine to standard therapy significantly improved outcomes in relapsed multiple myeloma patients, with notable increases in overall response rates .
Comparative Efficacy
Fludarabine's efficacy has been compared against other therapeutic agents, such as chlorambucil, particularly in elderly patients with CLL.
| Treatment | Overall Response Rate | Complete Remission Rate | Median Progression-Free Survival |
|---|---|---|---|
| Chlorambucil | 51% | 0% | 19 months |
| Fludarabine | 72% | 7% | 18 months |
These results indicate that while fludarabine may not significantly extend progression-free survival compared to chlorambucil, it does provide a higher overall response rate .
Safety Profile
While fludarabine is effective, it is associated with adverse effects including cytopenias and increased susceptibility to infections due to its immunosuppressive nature. Long-term studies have highlighted the need for careful monitoring during treatment.
生化学分析
Biochemical Properties
Fludarabine is a prodrug that is converted to the free nucleoside 9-β-D-arabinosyl-2-fluoroadenine (F-ara-A) which enters cells and accumulates mainly as the 5′-triphosphate, F-ara-ATP . The rate-limiting step in the formation of triphosphate is conversion of F-ara-A to its monophosphate, which is catalyzed by deoxycytidine kinase .
Cellular Effects
Fludarabine has been used in a variety of clinical circumstances, including use of fludarabine alone as well as in combinations with DNA-damaging agents or membrane-targeted antibodies . Other strategies have used fludarabine to reduce immunological function, thus facilitating non-myeloablative stem cell transplants .
Molecular Mechanism
Fludarabine exerts its effects at the molecular level through its active form, F-ara-ATP. It is involved in the inhibition of DNA synthesis by inhibition of ribonucleotide reductase, and by incorporation into DNA, leading to chain termination .
Metabolic Pathways
Fludarabine is involved in the nucleotide synthesis pathway. It is converted to its active form, F-ara-ATP, which then interacts with various enzymes and cofactors .
Transport and Distribution
Fludarabine monophosphate is dephosphorylated to the metabolite 9-β-arabinofuranosyl-2-fluoroadenine (F-Ara-A) within 5 minutes of intravenous infusion . F-Ara-A is then transported into the cell where it is converted to its active form, F-Ara-A-triphosphate (F-Ara-ATP) .
準備方法
Synthetic Routes and Reaction Conditions: Fludarabine can be synthesized using 2-fluoro-9-beta-D-(2’,3’,5’-tri-alkoxyarabinofuranosyl)adenine as a raw material. The reaction involves a sodium hydroxide and ammonia water mixed solution as a reagent and a water and 2-methyltetrahydrofuran mixed solution as a solvent. The reaction is carried out at 0-5°C for 1-3 hours, followed by neutralization with glacial acetic acid, vacuum filtration, recrystallization, and decolorization with activated carbon to obtain pure fludarabine .
Industrial Production Methods: Industrial production of fludarabine involves the esterification and hydrolysis of fludarabine and phosphorus oxychloride. The process includes coupling purine ring and sugar ring mother nucleus as initial materials, followed by multi-step operations and deprotection to obtain the target product .
化学反応の分析
Types of Reactions: Fludarabine undergoes various chemical reactions, including:
Oxidation: Fludarabine can be oxidized to form different metabolites.
Reduction: Reduction reactions can modify the functional groups on the fludarabine molecule.
Substitution: Substitution reactions can occur at the purine ring or sugar moiety.
Common Reagents and Conditions:
Oxidation: Common oxidizing agents include hydrogen peroxide and potassium permanganate.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Substitution reactions often involve nucleophiles like halides or amines under basic or acidic conditions.
Major Products: The major products formed from these reactions include various metabolites and derivatives of fludarabine, which can have different pharmacological properties .
類似化合物との比較
Clofarabine: Another purine nucleoside analog with similar mechanisms but higher toxicity in the upfront leukemia setting.
Bendamustine: Used as an alternative lymphodepleting regimen with potentially similar response rates and decreased toxicity.
Uniqueness: Fludarabine is unique due to its high selectivity for lymphocytes and its ability to inhibit DNA synthesis in both dividing and resting cells. Its immunosuppressive effects also make it valuable in conditioning regimens prior to stem cell transplantation .
生物活性
Fludarabine is a purine analog primarily used in the treatment of hematological malignancies, particularly chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma. Its biological activity is characterized by its ability to inhibit DNA synthesis, induce apoptosis, and exhibit antiviral properties. This article synthesizes findings from various studies to provide a comprehensive overview of the biological activity of fludarabine, supported by data tables and case studies.
Fludarabine is metabolized in the body to its active triphosphate form, which incorporates into DNA and RNA, thereby inhibiting further synthesis. The compound exerts its effects through several mechanisms:
- Inhibition of DNA Synthesis : Fludarabine inhibits ribonucleotide reductase, leading to a depletion of deoxynucleotide pools necessary for DNA repair and replication. This action enhances its incorporation into newly synthesized DNA, ultimately leading to cell death .
- Induction of Apoptosis : It triggers apoptosis by increasing pro-apoptotic proteins (e.g., Bax) while decreasing anti-apoptotic proteins (e.g., XIAP and survivin) .
- Antiviral Activity : Recent studies have shown that fludarabine exhibits broad-spectrum antiviral activity against RNA viruses such as Zika virus and SFTS phlebovirus. It inhibits viral RNA replication and protein expression in various cell types .
Efficacy in Clinical Trials
Fludarabine has been evaluated in multiple clinical trials for its efficacy in treating CLL and other hematological cancers. Below is a summary of key findings from notable studies:
| Study Type | Comparison | Response Rate | Progression-Free Survival | Overall Survival |
|---|---|---|---|---|
| First-line therapy vs Chlorambucil | Fludarabine (72%) vs Chlorambucil (51%) | Higher complete remission rate for fludarabine (7% vs 0%) | Similar (19 months for fludarabine vs 18 months for chlorambucil) | No significant difference (46 months vs 64 months) |
| Multicenter trial | Untreated patients | Fludarabine (71%) vs Control (60%) | Not specified | Not specified |
| Retrospective analysis | Pre-treated patients | Fludarabine (48%) vs Control (27%) | Not specified | Not specified |
These results indicate that while fludarabine may achieve higher response rates compared to chlorambucil, it does not significantly prolong overall survival or progression-free survival in certain patient populations .
Case Studies
- Chronic Lymphocytic Leukemia : In a cohort study involving elderly patients with CLL, fludarabine was administered as a first-line treatment. The study reported a complete remission rate of 7% and an overall response rate of 72%, suggesting that fludarabine may be particularly effective in early-stage disease but does not significantly improve outcomes in advanced stages .
- Combination Therapy : A study explored the combination of fludarabine with imatinib mesylate in patients with resistant forms of leukemia. The combination demonstrated synergistic effects, enhancing the overall response rates compared to monotherapy .
Antiviral Properties
Fludarabine's antiviral activity has garnered attention due to its potential application beyond oncology:
特性
IUPAC Name |
(2R,3S,4S,5R)-2-(6-amino-2-fluoropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6+,9-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HBUBKKRHXORPQB-FJFJXFQQSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=NC2=C(N=C(N=C2N1C3C(C(C(O3)CO)O)O)F)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=NC2=C(N=C(N=C2N1[C@H]3[C@H]([C@@H]([C@H](O3)CO)O)O)F)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H12FN5O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4039657 | |
| Record name | Fludarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4039657 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
285.23 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Sparingly sol water, org solvents | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. This metabolite appears to act by inhibiting DNA polymerase alpha, ribonucleotide reductase and DNA primase, thus inhibiting DNA synthesis. The mechanism of action of this antimetabolite is not completely characterized and may be multi-faceted., Fluorinated adenine analog causes inhibition of DNA synthesis by inhibiting ribonucleotide reductase & DNA polymerase., Fludarabine is a purine antimetabolite. Activity occurs as the result of activation to 2-fluoro-ara-ATP and includes inhibition of DNA synthesis (primarily in the S-phase of cell division by inhibition of ribonucleotide reductase and the DNA polymerases. It is also postulated that fludarabine interferes with RNA by decreased incorporation of uridine and leucine into RNA and protein, respectively. Fludarabine is also active against non-proliferating cells., This review establishes the pharmacokinetic characteristics of the major nucleoside analogs with cytotoxic activity. Cytarabine, pentostatin, fludarabine, cladribine & gemcitabine are all prodrugs whose plasma pharmacokinetics do not fully reflect their therapeutic activity; after cellular uptake, these compounds undergo phosphorylation by deoxycytidine kinase before their incorporation into DNA results in cell death. Cytarabine is principally active in the S phase of the cell cycle & is most toxic to replicating cells, whereas pentostatin, fludarabine & cladribine are incorporated into DNA during the process in which strand breaks are repaired & are therefore cytotoxic to slowly replicating cells (although the action of pentostatin results from its inhibition of adenosine deaminase). Gemcitabine is unusual in being highly metabolized in solid tumor cells. The cytotoxic activity of pentostatin, fludarabine and cladribine against the clonal cells of lymphoproliferative disorders is accompanied by damage to normal lymphoid cells, which results in significant & long-lasting immunosuppression. Useful interactions between nucleoside analogs have been defined. Cells that are primed by exposure to fludarabine or cladribine exhibit enhanced accumulation of cytarabine triphosphate (the cytotoxic nucleotide of cytarabine) & an improved therapeutic effect against acute myeloid leukemia & chronic lymphocytic leukemia can be achieved by clinical schedules that exploit this effect. Combinations of alkylating agents & fludarabine or cladribine are also synergistic in producing significantly enhanced activity against refractory lymphoid malignancies, but at the cost of increased hematological toxicity. Developments in the clinical admin of gemcitabine are concentrating on efforts to extend the duration of exposure to the drug as a means of counteracting its rapid catabolism in the circulation. Future developments with this group of agents will further explore the use of fludarabine-based combination therapies to produce a transient period of myelosuppression & immunosuppression that is sufficient to permit the engraftment of allogeneic hemopoietic stem cells & also exploit the immunological benefits of graft-versus-tumor reactions. In addition, the clinical spectrum of activity of gemcitabine is also being extended by combining the drug with other active chemotherapeutic agents, such as cisplatin, & by early studies of its role as a radiosensitiser. | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals | |
CAS No. |
21679-14-1 | |
| Record name | Fludarabine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=21679-14-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Fludarabine [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0021679141 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fludarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4039657 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Fludarabine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.040.462 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | FLUDARABINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/P2K93U8740 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
260 °C | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
















