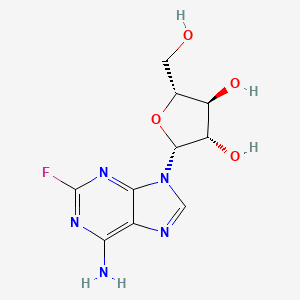
Fludarabina
Descripción general
Descripción
Fludarabina es un análogo de purina y un agente antineoplásicoEste compuesto se utiliza principalmente en el tratamiento de malignidades hematológicas como la leucemia linfocítica crónica, el linfoma no Hodgkin, la leucemia mieloide aguda y la leucemia linfocítica aguda . This compound funciona interfiriendo con la duplicación del ADN, lo que la convierte en un agente quimioterapéutico crucial .
Mecanismo De Acción
El fosfato de fludarabina se desfosforila rápidamente a 2-fluoro-ara-A y luego se fosforila intracelularmente por la desoxicitidina cinasa al trifosfato activo, 2-fluoro-ara-ATP. Este metabolito inhibe la ADN polimerasa alfa, la reductasa de ribonucleótidos y la primasa del ADN, lo que resulta en la inhibición de la síntesis de ADN y la destrucción de las células cancerosas .
Compuestos similares:
Clofarabina: Otro análogo de nucleósido de purina con mecanismos similares pero mayor toxicidad en la configuración de leucemia inicial.
Bendamustina: Se utiliza como un régimen de depleción linfocítica alternativo con tasas de respuesta potencialmente similares y menor toxicidad.
Singularidad: this compound es única debido a su alta selectividad para los linfocitos y su capacidad para inhibir la síntesis de ADN tanto en células en división como en reposo. Sus efectos inmunosupresores también la hacen valiosa en regímenes de acondicionamiento antes del trasplante de células madre .
Aplicaciones Científicas De Investigación
Chronic Lymphocytic Leukemia (CLL)
Fludarabine has become a standard treatment for CLL, particularly in patients with advanced disease or those who are refractory to previous therapies. The drug is often used alone or in combination with other agents like cyclophosphamide and rituximab.
Case Study: Fludarabine-Cyclophosphamide-Rituximab Regimen
- Patient Cohort : 300 patients with CLL
- Overall Response Rate : 95%
- Complete Remission : 72%
- Median Time to Progression : 80 months
- Survival Rates : Six-year overall survival was reported at 77% .
Allogeneic Stem Cell Transplantation
Fludarabine is frequently included in conditioning regimens prior to allogeneic stem cell transplantation due to its immunosuppressive effects, which facilitate engraftment and reduce the risk of graft-versus-host disease.
Research Findings:
- Fludarabine enhances the engraftment of human hematopoietic cells in immunocompromised mouse models.
- Studies indicate that fludarabine-treated mice exhibit improved levels of human hematopoiesis compared to controls .
Multiple Myeloma
In multiple myeloma, fludarabine has been studied as part of combination therapies aimed at improving response rates and prolonging survival.
Clinical Trial Results:
- A study demonstrated that the addition of fludarabine to standard therapy significantly improved outcomes in relapsed multiple myeloma patients, with notable increases in overall response rates .
Comparative Efficacy
Fludarabine's efficacy has been compared against other therapeutic agents, such as chlorambucil, particularly in elderly patients with CLL.
| Treatment | Overall Response Rate | Complete Remission Rate | Median Progression-Free Survival |
|---|---|---|---|
| Chlorambucil | 51% | 0% | 19 months |
| Fludarabine | 72% | 7% | 18 months |
These results indicate that while fludarabine may not significantly extend progression-free survival compared to chlorambucil, it does provide a higher overall response rate .
Safety Profile
While fludarabine is effective, it is associated with adverse effects including cytopenias and increased susceptibility to infections due to its immunosuppressive nature. Long-term studies have highlighted the need for careful monitoring during treatment.
Análisis Bioquímico
Biochemical Properties
Fludarabine is a prodrug that is converted to the free nucleoside 9-β-D-arabinosyl-2-fluoroadenine (F-ara-A) which enters cells and accumulates mainly as the 5′-triphosphate, F-ara-ATP . The rate-limiting step in the formation of triphosphate is conversion of F-ara-A to its monophosphate, which is catalyzed by deoxycytidine kinase .
Cellular Effects
Fludarabine has been used in a variety of clinical circumstances, including use of fludarabine alone as well as in combinations with DNA-damaging agents or membrane-targeted antibodies . Other strategies have used fludarabine to reduce immunological function, thus facilitating non-myeloablative stem cell transplants .
Molecular Mechanism
Fludarabine exerts its effects at the molecular level through its active form, F-ara-ATP. It is involved in the inhibition of DNA synthesis by inhibition of ribonucleotide reductase, and by incorporation into DNA, leading to chain termination .
Metabolic Pathways
Fludarabine is involved in the nucleotide synthesis pathway. It is converted to its active form, F-ara-ATP, which then interacts with various enzymes and cofactors .
Transport and Distribution
Fludarabine monophosphate is dephosphorylated to the metabolite 9-β-arabinofuranosyl-2-fluoroadenine (F-Ara-A) within 5 minutes of intravenous infusion . F-Ara-A is then transported into the cell where it is converted to its active form, F-Ara-A-triphosphate (F-Ara-ATP) .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: Fludarabina se puede sintetizar utilizando 2-fluoro-9-beta-D-(2’,3’,5’-tri-alcoxiarabinofuranosil)adenina como materia prima. La reacción implica una solución mixta de hidróxido de sodio y agua de amoníaco como reactivo y una solución mixta de agua y 2-metiltetrahidrofurano como solvente. La reacción se lleva a cabo a 0-5 °C durante 1-3 horas, seguida de neutralización con ácido acético glacial, filtración al vacío, recristalización y decoloración con carbón activado para obtener this compound pura .
Métodos de producción industrial: La producción industrial de this compound implica la esterificación e hidrólisis de this compound y oxicloruro de fósforo. El proceso incluye el acoplamiento del anillo de purina y el núcleo madre del anillo de azúcar como materiales iniciales, seguido de operaciones de varios pasos y desprotección para obtener el producto objetivo .
Análisis De Reacciones Químicas
Tipos de reacciones: Fludarabina experimenta varias reacciones químicas, que incluyen:
Oxidación: this compound se puede oxidar para formar diferentes metabolitos.
Reducción: Las reacciones de reducción pueden modificar los grupos funcionales en la molécula de this compound.
Sustitución: Las reacciones de sustitución pueden ocurrir en el anillo de purina o en la parte de azúcar.
Reactivos y condiciones comunes:
Oxidación: Los agentes oxidantes comunes incluyen peróxido de hidrógeno y permanganato de potasio.
Reducción: Se utilizan agentes reductores como borohidruro de sodio e hidruro de aluminio y litio.
Sustitución: Las reacciones de sustitución a menudo involucran nucleófilos como haluros o aminas en condiciones básicas o ácidas.
Productos principales: Los principales productos formados a partir de estas reacciones incluyen varios metabolitos y derivados de this compound, que pueden tener diferentes propiedades farmacológicas .
Comparación Con Compuestos Similares
Clofarabine: Another purine nucleoside analog with similar mechanisms but higher toxicity in the upfront leukemia setting.
Bendamustine: Used as an alternative lymphodepleting regimen with potentially similar response rates and decreased toxicity.
Uniqueness: Fludarabine is unique due to its high selectivity for lymphocytes and its ability to inhibit DNA synthesis in both dividing and resting cells. Its immunosuppressive effects also make it valuable in conditioning regimens prior to stem cell transplantation .
Actividad Biológica
Fludarabine is a purine analog primarily used in the treatment of hematological malignancies, particularly chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma. Its biological activity is characterized by its ability to inhibit DNA synthesis, induce apoptosis, and exhibit antiviral properties. This article synthesizes findings from various studies to provide a comprehensive overview of the biological activity of fludarabine, supported by data tables and case studies.
Fludarabine is metabolized in the body to its active triphosphate form, which incorporates into DNA and RNA, thereby inhibiting further synthesis. The compound exerts its effects through several mechanisms:
- Inhibition of DNA Synthesis : Fludarabine inhibits ribonucleotide reductase, leading to a depletion of deoxynucleotide pools necessary for DNA repair and replication. This action enhances its incorporation into newly synthesized DNA, ultimately leading to cell death .
- Induction of Apoptosis : It triggers apoptosis by increasing pro-apoptotic proteins (e.g., Bax) while decreasing anti-apoptotic proteins (e.g., XIAP and survivin) .
- Antiviral Activity : Recent studies have shown that fludarabine exhibits broad-spectrum antiviral activity against RNA viruses such as Zika virus and SFTS phlebovirus. It inhibits viral RNA replication and protein expression in various cell types .
Efficacy in Clinical Trials
Fludarabine has been evaluated in multiple clinical trials for its efficacy in treating CLL and other hematological cancers. Below is a summary of key findings from notable studies:
| Study Type | Comparison | Response Rate | Progression-Free Survival | Overall Survival |
|---|---|---|---|---|
| First-line therapy vs Chlorambucil | Fludarabine (72%) vs Chlorambucil (51%) | Higher complete remission rate for fludarabine (7% vs 0%) | Similar (19 months for fludarabine vs 18 months for chlorambucil) | No significant difference (46 months vs 64 months) |
| Multicenter trial | Untreated patients | Fludarabine (71%) vs Control (60%) | Not specified | Not specified |
| Retrospective analysis | Pre-treated patients | Fludarabine (48%) vs Control (27%) | Not specified | Not specified |
These results indicate that while fludarabine may achieve higher response rates compared to chlorambucil, it does not significantly prolong overall survival or progression-free survival in certain patient populations .
Case Studies
- Chronic Lymphocytic Leukemia : In a cohort study involving elderly patients with CLL, fludarabine was administered as a first-line treatment. The study reported a complete remission rate of 7% and an overall response rate of 72%, suggesting that fludarabine may be particularly effective in early-stage disease but does not significantly improve outcomes in advanced stages .
- Combination Therapy : A study explored the combination of fludarabine with imatinib mesylate in patients with resistant forms of leukemia. The combination demonstrated synergistic effects, enhancing the overall response rates compared to monotherapy .
Antiviral Properties
Fludarabine's antiviral activity has garnered attention due to its potential application beyond oncology:
Propiedades
IUPAC Name |
(2R,3S,4S,5R)-2-(6-amino-2-fluoropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6+,9-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HBUBKKRHXORPQB-FJFJXFQQSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=NC2=C(N=C(N=C2N1C3C(C(C(O3)CO)O)O)F)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=NC2=C(N=C(N=C2N1[C@H]3[C@H]([C@@H]([C@H](O3)CO)O)O)F)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H12FN5O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID4039657 | |
| Record name | Fludarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4039657 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
285.23 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Sparingly sol water, org solvents | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active triphosphate, 2-fluoro-ara-ATP. This metabolite appears to act by inhibiting DNA polymerase alpha, ribonucleotide reductase and DNA primase, thus inhibiting DNA synthesis. The mechanism of action of this antimetabolite is not completely characterized and may be multi-faceted., Fluorinated adenine analog causes inhibition of DNA synthesis by inhibiting ribonucleotide reductase & DNA polymerase., Fludarabine is a purine antimetabolite. Activity occurs as the result of activation to 2-fluoro-ara-ATP and includes inhibition of DNA synthesis (primarily in the S-phase of cell division by inhibition of ribonucleotide reductase and the DNA polymerases. It is also postulated that fludarabine interferes with RNA by decreased incorporation of uridine and leucine into RNA and protein, respectively. Fludarabine is also active against non-proliferating cells., This review establishes the pharmacokinetic characteristics of the major nucleoside analogs with cytotoxic activity. Cytarabine, pentostatin, fludarabine, cladribine & gemcitabine are all prodrugs whose plasma pharmacokinetics do not fully reflect their therapeutic activity; after cellular uptake, these compounds undergo phosphorylation by deoxycytidine kinase before their incorporation into DNA results in cell death. Cytarabine is principally active in the S phase of the cell cycle & is most toxic to replicating cells, whereas pentostatin, fludarabine & cladribine are incorporated into DNA during the process in which strand breaks are repaired & are therefore cytotoxic to slowly replicating cells (although the action of pentostatin results from its inhibition of adenosine deaminase). Gemcitabine is unusual in being highly metabolized in solid tumor cells. The cytotoxic activity of pentostatin, fludarabine and cladribine against the clonal cells of lymphoproliferative disorders is accompanied by damage to normal lymphoid cells, which results in significant & long-lasting immunosuppression. Useful interactions between nucleoside analogs have been defined. Cells that are primed by exposure to fludarabine or cladribine exhibit enhanced accumulation of cytarabine triphosphate (the cytotoxic nucleotide of cytarabine) & an improved therapeutic effect against acute myeloid leukemia & chronic lymphocytic leukemia can be achieved by clinical schedules that exploit this effect. Combinations of alkylating agents & fludarabine or cladribine are also synergistic in producing significantly enhanced activity against refractory lymphoid malignancies, but at the cost of increased hematological toxicity. Developments in the clinical admin of gemcitabine are concentrating on efforts to extend the duration of exposure to the drug as a means of counteracting its rapid catabolism in the circulation. Future developments with this group of agents will further explore the use of fludarabine-based combination therapies to produce a transient period of myelosuppression & immunosuppression that is sufficient to permit the engraftment of allogeneic hemopoietic stem cells & also exploit the immunological benefits of graft-versus-tumor reactions. In addition, the clinical spectrum of activity of gemcitabine is also being extended by combining the drug with other active chemotherapeutic agents, such as cisplatin, & by early studies of its role as a radiosensitiser. | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals | |
CAS No. |
21679-14-1 | |
| Record name | Fludarabine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=21679-14-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Fludarabine [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0021679141 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Fludarabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4039657 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Fludarabine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.040.462 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | FLUDARABINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/P2K93U8740 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
260 °C | |
| Record name | Fludarabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01073 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | FLUDARABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6964 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















