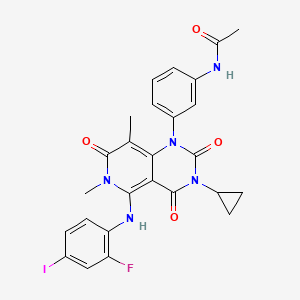
トラメチニブ
概要
説明
トラメチニブは、経口生物学的利用能のあるミトゲン活性化タンパク質キナーゼ(MAPK)キナーゼ(MEK)阻害剤であり、抗腫瘍活性があります。 MEK1およびMEK2に特異的に結合し、さまざまな癌における成長因子媒介細胞シグナル伝達および細胞増殖を阻害します . トラメチニブは、主にBRAF V600EまたはV600K変異を伴う切除不能または転移性黒色腫の治療に使用されます .
科学的研究の応用
Clinical Applications in Oncology
Trametinib has been extensively studied in clinical settings, particularly for its role in treating cancers associated with BRAF mutations.
Melanoma Treatment
Trametinib is approved for use in patients with BRAF V600E or V600K mutation-positive metastatic melanoma. In clinical trials, trametinib has shown significant efficacy:
- Phase II Studies : One study reported a 33% confirmed response rate among patients with BRAF-mutant melanoma, highlighting its ability to decrease cell proliferation and induce apoptosis .
- Combination Therapy : The combination of trametinib with dabrafenib (another BRAF inhibitor) has been shown to improve outcomes significantly compared to monotherapy, enhancing overall response rates and progression-free survival .
Ovarian Cancer
Recent studies have demonstrated trametinib's effectiveness in treating low-grade serous ovarian cancer:
- Randomized Trials : In a phase II/III trial, trametinib significantly increased progression-free survival compared to standard therapies, establishing it as a new standard of care for recurrent low-grade serous ovarian cancer .
Other Cancers
Trametinib has shown promise in various other malignancies:
- Non-Small Cell Lung Cancer : Research indicates that trametinib can be effective in patients with non-small cell lung cancer harboring BRAF mutations .
- Pediatric Gliomas : The combination of trametinib with dabrafenib has outperformed traditional chemotherapy in pediatric patients with BRAF V600E mutation-positive low-grade gliomas, leading to better tumor control and fewer side effects .
Preclinical Research and Emerging Applications
Beyond established clinical uses, trametinib is being investigated for potential applications in other areas:
Kidney Fibrosis
Recent studies suggest that trametinib may have antifibrotic effects in chronic kidney disease models. It has been shown to reduce collagen deposition and myofibroblast differentiation in animal models of renal fibrosis, indicating a potential role in treating fibrotic diseases beyond oncology .
Other Solid Tumors
There is ongoing research into the efficacy of trametinib against solid tumors with various genetic backgrounds, including those lacking typical BRAF mutations but still exhibiting MEK pathway activation .
Safety Profile and Adverse Effects
While trametinib is generally well-tolerated, it is associated with several adverse effects:
- Common side effects include skin rash, diarrhea, fatigue, peripheral edema, and nausea .
- Serious adverse events can include ocular issues (e.g., retinal vein occlusion) and cardiovascular concerns (e.g., decreased left ventricular ejection fraction) .
Case Studies and Clinical Trials
The following table summarizes key clinical trials involving trametinib:
| Study | Population | Treatment | Outcomes |
|---|---|---|---|
| NCT00687622 | Patients with solid tumors | Trametinib | 33% response rate in BRAF-mutant melanoma |
| NRG-GOG 0281/LOGS | Women with recurrent low-grade serous ovarian cancer | Trametinib vs Standard Care | Increased progression-free survival (13 months vs 7 months) |
| Phase II Trial | Pediatric glioma patients | Dabrafenib + Trametinib | Superior tumor shrinkage compared to chemotherapy |
作用機序
トラメチニブは、MAPK/ERKシグナル伝達経路の主要な構成要素であるMEK1およびMEK2の活性を阻害することで作用します . この経路は、細胞増殖、生存、分化、運動性の調節に関与しています . MEK1およびMEK2を阻害することで、トラメチニブは下流シグナル伝達分子のリン酸化と活性化を阻止し、腫瘍細胞の増殖を阻害します .
類似化合物の比較
類似化合物
コビメチニブ: 黒色腫の治療のためにベムラフェニブと組み合わせて使用される別のMEK阻害剤.
セルメチニブ: 神経線維腫症1型の治療に使用されるMEK阻害剤.
トラメチニブの独自性
トラメチニブは、MEK1とMEK2の両方を高い効力で選択的に阻害できる点で独特です . ダブラフェニブなどのBRAF阻害剤と組み合わせて、BRAF変異癌の治療に有意な有効性が示されています . この併用療法は、いずれかの薬剤単独療法よりも腫瘍増殖の阻害効果が大きく、持続的なことが示されています .
生化学分析
Biochemical Properties
Trametinib specifically binds to MEK1 and MEK2, resulting in inhibition of growth factor-mediated cell signaling and cellular proliferation in various cancers . It is a reversible, highly selective, allosteric inhibitor of MEK1 and MEK2 . By binding to unphosphorylated MEK1 and MEK2 with high affinity, trametinib blocks the catalytic activity of MEKs .
Cellular Effects
Trametinib has been shown to inhibit the proliferation, migration, and invasion of glioma cells, while inducing apoptosis of glioma cells . It can suppress both the expression of PKM2 in glioma cells and the transport of PKM2 into the cellular nucleus via suppression of ERK1/2 expression . Trametinib also significantly reduces the phosphorylation of MEK1/2 and extracellular signal-regulated kinase 1/2 (ERK1/2), mitigated renal dysfunction, and ameliorated histopathological abnormalities .
Molecular Mechanism
Trametinib is a kinase inhibitor that inhibits cell growth of various BRAF V600 mutation-positive tumors in vitro and in vivo . It functions as an allosteric, ATP noncompetitive inhibitor with nanomolar activity against both MEK 1 and MEK 2 kinases . It maintains MEK in an unphosphorylated form, preventing phosphorylation and activation of MEKs .
Temporal Effects in Laboratory Settings
Trametinib has been shown to have significant effects over time in laboratory settings. For instance, it has been found that trametinib can inhibit the growth and intracellular glycolysis of glioma cells by targeting the PKM2/c-Myc pathway . Moreover, trametinib treatment accelerated disease onset and decreased epidermal thickness, which was in large part ameliorated by Losartan treatment .
Dosage Effects in Animal Models
The effects of trametinib vary with different dosages in animal models. For instance, trametinib has been shown to significantly enhance remyelination in both MOG-induced EAE model and LPC-induced focal demyelination model . Furthermore, trametinib has been shown to inhibit the growth of the transplanted glioma cell tumor .
Metabolic Pathways
Trametinib is involved in the MAPK pathway, which plays a critical role in cell growth, differentiation, inflammation, and apoptosis . Mutant BRAF proteins signal through MEK1 and MEK2, stimulating cell growth . Trametinib is metabolized predominantly via deacetylation followed by oxidation and/or glucuronidation .
Transport and Distribution
Trametinib has limited brain distribution due to active efflux at the blood-brain barrier (BBB) . Following administration, trametinib and its metabolites are excreted in the feces (≥81%) and to a minor extent in urine (≤19%) .
Subcellular Localization
Trametinib is mainly localized in the cytoplasm . It has been shown that trametinib can suppress both the expression of PKM2 in glioma cells and the transport of PKM2 into the cellular nucleus via suppression of ERK1/2 expression .
準備方法
合成経路および反応条件
トラメチニブの合成には、いくつかの重要なステップが含まれます。 出発物質は一般的に置換アニリンであり、環化、アシル化、ハロゲン化などの反応を複数回行うことで最終生成物が得られます . 反応条件には、ジメチルスルホキシドなどの有機溶媒とチオニルクロリドなどの試薬の使用がしばしば含まれます .
工業生産方法
工業用では、トラメチニブの生産は、同様の合成経路を使用してスケールアップされていますが、収率と純度を高くするために反応条件が最適化されています。 プロセスには、不純物の生成を監視し、最終製品が規制基準を満たすようにするための厳しい品質管理対策が含まれます .
化学反応の分析
反応の種類
トラメチニブは、以下を含むさまざまな化学反応を受けます。
酸化: トラメチニブは特定の条件下で酸化され、分解生成物を生成する可能性があります.
還元: 還元反応も起こり得ますが、それほど一般的ではありません。
一般的な試薬および条件
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムが含まれます。
還元: 水素化ホウ素ナトリウムなどの還元剤を使用できます。
生成される主要な生成物
これらの反応から生成される主要な生成物には、脱アセチルトラメチニブやシクロプロパンアミド不純物などのさまざまな中間体や不純物が含まれます .
科学研究への応用
トラメチニブは、幅広い科学研究用途があります。
類似化合物との比較
Similar Compounds
Cobimetinib: Another MEK inhibitor used in combination with vemurafenib for the treatment of melanoma.
Selumetinib: A MEK inhibitor used for the treatment of neurofibromatosis type 1.
Uniqueness of Trametinib
Trametinib is unique in its ability to selectively inhibit both MEK1 and MEK2 with high potency . It has shown significant efficacy in combination with BRAF inhibitors, such as dabrafenib, for the treatment of BRAF-mutant cancers . This combination therapy has been shown to provide greater and more prolonged inhibition of tumor growth compared to either drug alone .
生物活性
Trametinib is a potent, selective allosteric inhibitor of MEK1 and MEK2, primarily utilized in the treatment of cancers associated with BRAF mutations, particularly melanoma. This article explores the biological activity of trametinib, including its mechanisms of action, pharmacodynamics, clinical efficacy, and safety profile.
Trametinib functions by inhibiting the mitogen-activated protein kinase (MAPK) pathway, which is often dysregulated in cancer due to mutations in the BRAF gene. BRAF mutations lead to constitutive activation of the RAS-RAF-MEK-ERK signaling cascade, promoting uncontrolled cell proliferation and survival. By binding to unphosphorylated MEK1 and MEK2, trametinib effectively prevents their activation and subsequent phosphorylation of ERK1/2:
- Inhibition Mechanism : Trametinib exhibits a half-maximum inhibitory concentration (IC50) of 0.7–0.9 nmol/L for MEK1/2, showing high selectivity as it does not inhibit over 98 other kinases tested .
- Biomarkers : The drug's efficacy can be monitored through biomarkers such as phosphorylated ERK (p-ERK), Ki-67, and p27 .
Pharmacodynamics
Trametinib demonstrates a favorable pharmacokinetic profile:
- Absorption : Rapid absorption with a median time to maximum plasma concentration (Tmax) of 1.5 hours after oral administration.
- Bioavailability : Approximately 72% after a single dose.
- Half-Life : A terminal half-life of about 5.3 days allows for sustained drug action with daily dosing .
Clinical Efficacy
Trametinib has shown substantial clinical activity in various cancers:
Melanoma
A pivotal study compared trametinib with chemotherapy in patients with advanced melanoma harboring BRAF mutations:
| Treatment | Median Time to Progression | Overall Response Rate | Survival Rate (6 months) |
|---|---|---|---|
| Trametinib | ~5 months | 22% | 81% |
| Chemotherapy | 1.5 months | 8% | 67% |
The results indicated that trametinib significantly outperformed chemotherapy in both progression-free survival and overall survival .
Glioma
In pediatric glioma patients with BRAF V600 mutations, a combination of dabrafenib and trametinib was found to be superior to traditional chemotherapy:
- Outcome Improvement : The combination therapy shrank tumors more effectively and maintained tumor control for nearly three times longer than chemotherapy .
Low-Grade Serous Ovarian Cancer
A randomized trial demonstrated that trametinib significantly improved progression-free survival compared to standard care:
| Group | Median Progression-Free Survival |
|---|---|
| Trametinib | 13.0 months |
| Standard Care | 7.2 months |
This trial established trametinib as a new standard-of-care option for this cancer type .
Safety Profile
While trametinib is generally well-tolerated, it is associated with specific side effects:
- Common Adverse Events : Rash, diarrhea, fatigue, and elevated liver enzymes.
- Cardiotoxicity : Rare instances of cardiomyopathy have been reported, necessitating monitoring of cardiac function during treatment. About 11% of patients may experience decreased ejection fraction (EF) related to trametinib therapy .
Case Study 1: Advanced Melanoma
A patient with BRAF V600E mutation treated with trametinib experienced significant tumor reduction with minimal side effects compared to prior chemotherapy.
Case Study 2: Pediatric Glioma
A child diagnosed with recurrent glioma showed remarkable tumor shrinkage after receiving the combination therapy of dabrafenib and trametinib, leading to prolonged remission.
特性
IUPAC Name |
N-[3-[3-cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxopyrido[4,3-d]pyrimidin-1-yl]phenyl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C26H23FIN5O4/c1-13-22-21(23(31(3)24(13)35)30-20-10-7-15(28)11-19(20)27)25(36)33(17-8-9-17)26(37)32(22)18-6-4-5-16(12-18)29-14(2)34/h4-7,10-12,17,30H,8-9H2,1-3H3,(H,29,34) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LIRYPHYGHXZJBZ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C2C(=C(N(C1=O)C)NC3=C(C=C(C=C3)I)F)C(=O)N(C(=O)N2C4=CC=CC(=C4)NC(=O)C)C5CC5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C26H23FIN5O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID901007381 | |
| Record name | N-{3-[3-Cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}ethanimidic acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID901007381 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
615.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Trametinib is a reversible, allosteric inhibitor of mitogen-activated extracellular signal regulated kinase 1 _(MEK1)_ and _MEK2_ activation and of_ MEK1_ and _MEK2_ kinase activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. Trametinib helps with melanoma with the BRAF V600E or V600K as the mutation results in the constitutive activation of the BRAF pathway which includes MEK1 and MEK2. | |
| Record name | Trametinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08911 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
871700-17-3 | |
| Record name | Trametinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=871700-17-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Trametinib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0871700173 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Trametinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08911 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | N-{3-[3-Cyclopropyl-5-(2-fluoro-4-iodoanilino)-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}ethanimidic acid | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID901007381 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-{3-[3-cyclopropyl-5-[(2-fluoro-4-iodophenyl)amino]-6,8-dimethyl-2,4,7-trioxo-3,4,6,7-tetrahydropyrido[4,3-d]pyrimidin-1(2H)-yl]phenyl}acetamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | TRAMETINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/33E86K87QN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
293-303 | |
| Record name | Trametinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08911 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。













