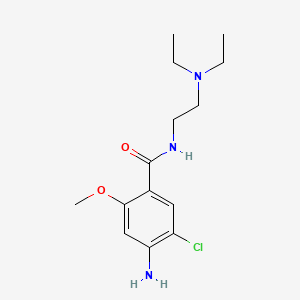
Metoclopramid
Übersicht
Beschreibung
Metoclopramid ist ein Medikament, das in erster Linie zur Behandlung von Übelkeit und Erbrechen sowie zur Erleichterung der Magenentleerung bei Patienten mit verzögerter Magenentleerung eingesetzt wird. Es wird auch zur Behandlung von gastroösophagealer Refluxkrankheit und Migränekopfschmerzen eingesetzt .
Wirkmechanismus
Target of Action
Metoclopramide primarily targets dopamine D2 receptors and serotonin 5-HT3 and 5-HT4 receptors . These receptors play a crucial role in the regulation of nausea, vomiting, and gastric motility .
Mode of Action
Metoclopramide acts as an antagonist at dopamine D2 receptors and serotonin 5-HT3 receptors, and as an agonist at serotonin 5-HT4 receptors . By inhibiting dopamine D2 receptors, it interferes with the central mechanisms that create the sensation of nausea and also stimulates lower oesophageal sphincter contraction and gastric motility . Its action on serotonin receptors further enhances its antiemetic and prokinetic effects .
Biochemical Pathways
The biochemical pathways affected by metoclopramide primarily involve the dopaminergic and serotonergic systems . By blocking dopamine D2 receptors, metoclopramide interferes with dopaminergic neurotransmission in the chemoreceptor trigger zone (CTZ) located in the area postrema of the brain, which is involved in the sensation of nausea and vomiting . Its action on serotonin receptors further modulates these effects .
Pharmacokinetics
Metoclopramide is rapidly and well absorbed from the gastrointestinal tract, with an oral bioavailability of 80 ± 15% . It undergoes first-pass metabolism in the liver, primarily through the cytochrome P450 CYP2D6 pathway . The major metabolites of metoclopramide are produced via N-hydroxylation and N-deethylation . The elimination half-life of metoclopramide is dose-dependent, ranging from 5 to 6 hours . Approximately 70-85% of the drug is excreted in the urine .
Result of Action
The molecular and cellular effects of metoclopramide’s action result in its antiemetic and prokinetic properties. It relieves symptoms such as nausea, vomiting, heartburn, a feeling of fullness after meals, and loss of appetite . It is also used to stimulate gastric emptying in patients with delayed stomach emptying .
Action Environment
The action, efficacy, and stability of metoclopramide can be influenced by various environmental factors. For instance, the presence of certain medical conditions, such as diabetic gastroparesis and gastroesophageal reflux disease (GERD), can affect the drug’s action . Additionally, the drug’s metabolism can be affected by genetic polymorphisms in the CYP2D6 enzyme , potentially influencing its efficacy and side effect profile.
Wissenschaftliche Forschungsanwendungen
Pharmacological Overview
Metoclopramide acts primarily as an antagonist at dopamine D2 receptors and has additional effects on serotonin receptors (5HT3 and 5HT4). This dual action enhances gastrointestinal motility and reduces nausea and vomiting by blocking signals in the chemoreceptor trigger zone of the brain.
Clinical Applications
Metoclopramide is utilized in several clinical scenarios:
-
Gastrointestinal Disorders
- Diabetic Gastroparesis : Approved for the treatment of diabetic gastroparesis, metoclopramide improves gastric emptying and alleviates symptoms like nausea and vomiting .
- Gastroesophageal Reflux Disease (GERD) : It is indicated for patients who do not respond to conventional therapies, helping to reduce reflux symptoms .
-
Nausea and Vomiting
- Chemotherapy-Induced Nausea and Vomiting (CINV) : Metoclopramide is used to prevent nausea associated with chemotherapy, although it may be less effective than other agents like 5-HT3 antagonists .
- Postoperative Nausea and Vomiting : It is administered to prevent nausea following surgery when nasogastric suction is not feasible .
- Off-Label Uses
Case Study 1: Diabetic Gastroparesis
A multicenter placebo-controlled trial demonstrated that metoclopramide significantly reduced symptoms of nausea, vomiting, and postprandial fullness in patients with diabetic gastroparesis. The study involved 40 patients over three weeks, showing statistically significant improvements compared to placebo .
Case Study 2: Acute Dystonia
A recent report highlighted two adolescents who developed acute dystonia after metoclopramide administration. Both cases involved symptoms such as muscle spasms and abnormal eye movements, underscoring the need for careful monitoring of extrapyramidal side effects associated with this medication .
Case Study 3: Pregnancy Safety
A meta-analysis involving over 33,000 pregnant women found no significant association between first-trimester metoclopramide use and major congenital malformations, supporting its safety during early pregnancy .
Summary of Findings
The applications of metoclopramide are extensive, particularly in managing gastrointestinal disorders and preventing nausea in various clinical settings. However, awareness of potential side effects, including acute dystonia and tardive dyskinesia with prolonged use, is crucial for safe administration.
Data Table: Summary of Clinical Applications
Biochemische Analyse
Biochemical Properties
Metoclopramide interacts with various enzymes and proteins, primarily through its antagonistic effects on dopamine D2 and serotonin 5-HT3 receptors . It also exhibits agonistic effects on serotonin 5-HT4 receptors and antagonizes muscarinic receptor inhibition . These interactions play a crucial role in its function in biochemical reactions .
Cellular Effects
Metoclopramide has significant effects on various types of cells and cellular processes. It influences cell function by inhibiting dopamine D2 and serotonin 5-HT3 receptors in the chemoreceptor trigger zone (CTZ) located in the area postrema of the brain . This leads to prokinetic effects via inhibitory actions on presynaptic and postsynaptic D2 receptors, agonism of serotonin 5-HT4 receptors, and antagonism of muscarinic receptor inhibition .
Molecular Mechanism
Metoclopramide exerts its effects at the molecular level through several mechanisms. It inhibits dopamine D2 and serotonin 5-HT3 receptors in the chemoreceptor trigger zone (CTZ) of the brain . This leads to prokinetic effects via inhibitory actions on presynaptic and postsynaptic D2 receptors, agonism of serotonin 5-HT4 receptors, and antagonism of muscarinic receptor inhibition .
Temporal Effects in Laboratory Settings
The effects of Metoclopramide can change over time in laboratory settings. The risk of acute neurological effects is higher in children than in adults . The review has confirmed a well‐established safety profile for metoclopramide, including the risks of neurological adverse effects (e.g. acute extrapyramidal symptoms and irreversible tardive dyskinesia) .
Dosage Effects in Animal Models
The effects of Metoclopramide can vary with different dosages in animal models. The dosage must be adapted in animals with renal or hepatic insufficiency due to an increase in the risk of side effects . The dosage should be carefully observed, especially in cats and small breed dogs .
Metabolic Pathways
Metoclopramide undergoes first-pass metabolism and its metabolism varies according to the individual . This drug is metabolized by cytochrome P450 enzymes in the liver . CYP2D6 and CYP3A4 both contribute to its metabolism, with CYP2D6 being more heavily involved .
Transport and Distribution
The volume of distribution of Metoclopramide is approximately 3.5 L/kg . This implies a high level of tissue distribution. Metoclopramide crosses the placental barrier and can cause extrapyramidal symptoms in the fetus .
Subcellular Localization
Given its mechanism of action, it is likely to be found in areas where dopamine D2 and serotonin 5-HT3 receptors are present, such as the chemoreceptor trigger zone (CTZ) located in the area postrema of the brain .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen
Metoclopramid kann durch verschiedene Verfahren synthetisiert werden. Eine gängige Methode beinhaltet die Reaktion von 4-Amino-5-Chlor-2-Methoxybenzoesäure mit N,N-Diethyl-2-Chlorethylamin in Gegenwart einer Base, um die Zwischenverbindung zu bilden, die dann durch weitere Reaktionen zu this compound umgewandelt wird .
Industrielle Produktionsmethoden
Die industrielle Produktion von this compound beinhaltet in der Regel die Herstellung von Metoclopramidhydrochlorid. Das Verfahren beinhaltet das Auflösen von this compound in einer Weinsäurelösung, gefolgt von der Zugabe von Natriumpyrosulfit und Natriumacetatlösung zur Einstellung des pH-Werts. Die Lösung wird dann filtriert und verschiedenen Reinigungsschritten unterzogen, um das Endprodukt zu erhalten .
Analyse Chemischer Reaktionen
Arten von Reaktionen
Metoclopramid durchläuft verschiedene Arten von chemischen Reaktionen, darunter:
Oxidation: this compound kann oxidiert werden, um verschiedene Metaboliten zu bilden.
Reduktion: Reduktionsreaktionen können die in this compound vorhandenen funktionellen Gruppen verändern.
Substitution: Substitutionsreaktionen können am aromatischen Ring oder an anderen funktionellen Gruppen auftreten.
Häufige Reagenzien und Bedingungen
Häufige Reagenzien, die in den Reaktionen mit this compound verwendet werden, umfassen Oxidationsmittel wie Wasserstoffperoxid, Reduktionsmittel wie Natriumborhydrid und verschiedene Säuren und Basen für Substitutionsreaktionen .
Hauptprodukte, die gebildet werden
Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, umfassen verschiedene Metaboliten und Derivate von this compound, die unterschiedliche pharmakologische Eigenschaften haben können .
Vergleich Mit ähnlichen Verbindungen
Ähnliche Verbindungen
Einige Verbindungen, die Metoclopramid ähneln, sind:
Domperidon: Ein weiterer Dopaminrezeptorantagonist, der zur Behandlung von Übelkeit und Erbrechen eingesetzt wird.
Prochlorperazin: Wird zur Behandlung von Übelkeit, Erbrechen und Migränekopfschmerzen eingesetzt.
Chlorpromazin: Ein Antipsychotikum mit emetischen Eigenschaften.
Einzigartigkeit von this compound
This compound ist einzigartig in seiner Kombination aus Dopamin-D2-Rezeptorantagonismus und 5-HT3-Rezeptorantagonismus/5-HT4-Rezeptoragonismus, was ein breites Spektrum an prokinetischen und emetischen Wirkungen bietet. Dadurch ist es besonders effektiv bei der Behandlung von Erkrankungen wie Gastroparese und schwerer Übelkeit .
Biologische Aktivität
Metoclopramide is a widely used medication primarily known for its antiemetic properties. It functions as a dopamine D2 receptor antagonist and has significant effects on gastrointestinal motility. This article delves into its biological activity, mechanisms of action, pharmacokinetics, clinical applications, and associated case studies.
Metoclopramide exerts its effects through multiple pathways:
- Dopamine Receptor Antagonism : It primarily antagonizes dopamine D2 receptors in the chemoreceptor trigger zone (CTZ) of the brain, which reduces nausea and vomiting by inhibiting signals that trigger these sensations .
- Serotonin Receptor Interaction : Metoclopramide also acts as an antagonist at 5-HT3 receptors and an agonist at 5-HT4 receptors. This dual action enhances gastrointestinal motility by increasing acetylcholine release, thereby improving lower esophageal sphincter tone and gastric emptying .
- Gastrointestinal Motility : The drug promotes gastric emptying without significantly increasing secretions from the biliary, gastric, or pancreatic systems. This property makes it effective in treating conditions like diabetic gastroparesis .
Pharmacokinetics
The pharmacokinetic profile of metoclopramide includes:
Clinical Applications
Metoclopramide is indicated for various conditions, including:
- Nausea and Vomiting : Particularly effective in gastroesophageal reflux disease (GERD) and chemotherapy-induced nausea and vomiting (CINV) .
- Gastroparesis : Shown to improve symptoms such as nausea, vomiting, and early satiety in diabetic patients .
- Chronic Intractable Hiccups (CIH) : Some studies suggest its effectiveness in managing CIH, although more data is needed for conclusive evidence .
Adverse Effects
Despite its benefits, metoclopramide can cause various side effects due to its antidopaminergic activity:
- Extrapyramidal Symptoms : These include tardive dyskinesia, acute dystonia, and akathisia, particularly with prolonged use or high doses .
- Other Side Effects : Commonly reported adverse effects include drowsiness, diarrhea, hypotension, and movement disorders .
Case Study 1: Effectiveness in CIH
A retrospective study involving 96 patients evaluated the long-term effectiveness of metoclopramide for CIH. Out of 14 eligible patients, six continued treatment for an average of 27 months with notable improvements on various clinical scales. However, eight patients discontinued treatment after a mean duration of eight months due to side effects .
Case Study 2: Acute Dystonic Reaction
A case report described a 17-year-old male who developed acute dystonia after receiving metoclopramide for gastroenteritis. Symptoms included facial grimacing and muscle spasms following administration of the drug. The reaction was resolved with diazepam, highlighting the potential for acute adverse effects even with standard dosing regimens .
Eigenschaften
IUPAC Name |
4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H22ClN3O2/c1-4-18(5-2)7-6-17-14(19)10-8-11(15)12(16)9-13(10)20-3/h8-9H,4-7,16H2,1-3H3,(H,17,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
TTWJBBZEZQICBI-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CC)CCNC(=O)C1=CC(=C(C=C1OC)N)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H22ClN3O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
2576-84-3 (di-hydrochloride), 54143-57-6 (mono-hydrochloride, mono-hydrate), 7232-21-5 (mono-hydrochloride) | |
| Record name | Metoclopramide [INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000364625 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID6045169 | |
| Record name | Metoclopramide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6045169 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
299.79 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Metoclopramide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Crystal; decomposes at 145 °C. Solubility at 25 °C (g/100 mL): water 48; ethanol (95%) 9; absolute ethanol 6; benzene 0.10; chloroform 0.10. Stable in acidic solutions. Unstable in strongly alkaline solutions. /Metoclopramide Dihydrochloride monohydrate/, Solubility at 25 °C (g/100 mL): 95% ethanol 2.30; absolute ethanol 1.90; benzene 0.10; chloroform 6.60, In water, 0.02 g/100 mL at 25 °C, 3.10e-01 g/L | |
| Record name | Metoclopramide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Metoclopramide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Metoclopramide causes antiemetic effects by inhibiting dopamine D2 and serotonin 5-HT3 receptors in the chemoreceptor trigger zone (CTZ) located in the area postrema of the brain. Administration of this drug leads to prokinetic effects via inhibitory actions on presynaptic and postsynaptic D2 receptors, agonism of serotonin 5-HT4 receptors, and antagonism of muscarinic receptor inhibition. This action enhances the release of acetylcholine, causing increased lower esophageal sphincter (LES) and gastric tone, accelerating gastric emptying and transit through the gut. Metoclopramide antagonizes the dopamine D2 receptors. Dopamine exerts relaxant effect on the gastrointestinal tract through binding to muscular D2 receptors., Metoclopramide accelerates gastric emptying and intestinal transit from the duodenum to the ileocecal valve by increasing the amplitude and duration of esophageal contractions, the resting tone of the lower esophageal sphincter, and the amplitude and tone of gastric (especially antral) contractions and by relaxing the pyloric sphincter and the duodenal bulb, while increasing peristalsis of the duodenum and jejunum. Unlike nonspecific cholinergic-like stimulation of upper GI smooth muscle, the stimulant effects of metoclopramide on GI smooth muscle coordinate gastric, pyloric, and duodenal motor activity., The pharmacologic actions of metoclopramide on the upper GI tract are similar to those of cholinergic drugs (e.g., bethanechol); however, unlike cholinergic drugs, metoclopramide does not stimulate gastric, biliary, or pancreatic secretions and does not affect serum gastrin concentration. Although the exact mechanism of action of metoclopramide is unclear, the effects of metoclopramide on GI motility may be mediated via enhancement of cholinergic excitatory processes at the postganglionic neuromuscular junction; antagonism of nonadrenergic, noncholinergic inhibitory motor nerves (i.e., dopaminergic); and/or a direct effect on smooth muscle., The effects of metoclopramide on GI motility do not depend on intact vagal innervations but are reduced or abolished by anticholinergic drugs (e.g., atropine) and potentiated by cholinergic drugs (e.g., carbachol, methacholine). These findings suggest that metoclopramide's effects on GI motility may depend in part on intramural cholinergic neurons of smooth muscle that are intact after vagal denervation. Unlike cholinergic drugs, metoclopramide requires intrinsic neuronal storage sites of acetylcholine to exert its pharmacologic effects. Postsynaptic activity results from metoclopramide's ability to enhance release of acetylcholine from postganglionic cholinergic neurons in the GI tract and to sensitize muscarinic receptors of GI smooth muscle to the actions of acetylcholine., Metoclopramide is a potent dopamine-receptor antagonist, and some of the actions of metoclopramide on GI smooth muscle may be mediated via antagonism of dopaminergic neurotransmission, Specific dopamine receptors in the esophagus and stomach have been identified; however, it is not known if there is a dopaminergic control system for smooth muscle function in the upper GI tract. In the GI tract, dopamine is principally an inhibitory neurotransmitter. Dopamine decreases the intensity of esophageal contractions, relaxes the proximal stomach, and reduces gastric secretion. Although metoclopramide blocks these inhibitory effects of dopamine, the actual role of dopamine in the peripheral control of GI motility has not been fully elucidated. Since cholinergic mechanisms are responsible for most excitatory motor activity in the GI tract, it appears that metoclopramide's therapeutic effects are principally caused by the drug's cholinergic-like activity; however, antagonism of GI dopaminergic activity may augment metoclopramide's cholinergic-like activity., For more Mechanism of Action (Complete) data for Metoclopramide (10 total), please visit the HSDB record page. | |
| Record name | Metoclopramide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01233 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Metoclopramide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
364-62-5 | |
| Record name | Metoclopramide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=364-62-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Metoclopramide [INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000364625 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Metoclopramide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01233 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Metoclopramide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6045169 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Metoclopramide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.006.058 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | METOCLOPRAMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/L4YEB44I46 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Metoclopramide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Metoclopramide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
171-173, 146.5-148 °C, 147.25 °C | |
| Record name | Metoclopramide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01233 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Metoclopramide | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7841 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Metoclopramide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.













