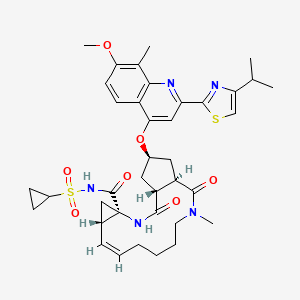
Simeprevir
Übersicht
Beschreibung
TMC435, auch bekannt als Simeprevir, ist ein potenter, einmal täglich einzunehmender, nicht-kovalenter, oraler Hepatitis-C-Virus-NS3/4A-Protease-Inhibitor. Es wird in Kombination mit pegyliertem Interferon und Ribavirin zur Behandlung chronischer Hepatitis-C-Virus-Infektionen des Genotyps 1 eingesetzt. Dieser Wirkstoff wurde in mehreren Ländern zur Anwendung zugelassen, darunter Japan, Kanada, die Vereinigten Staaten, Russland, die Europäische Union, Mexiko und Australien .
Wirkmechanismus
Target of Action
Simeprevir is a direct-acting antiviral agent that primarily targets the Hepatitis C Virus (HCV) NS3/4A protease . This protease is essential for the viral replication process of HCV, particularly for genotypes 1 and 4 .
Mode of Action
This compound inhibits the HCV NS3/4A protease in a potent and highly specific manner . This protease is responsible for cleaving the HCV-encoded polyprotein into individual viral proteins, which is a critical step in the HCV viral life cycle . By inhibiting this protease, this compound effectively blocks the viral replication process .
Biochemical Pathways
The inhibition of the HCV NS3/4A protease disrupts the viral replication process, preventing the maturation of the virus . This compound also shows synergistic effects with interferon-α and HCV NS5B inhibitor, and additive effects with ribavirin in HCV replicon cells .
Pharmacokinetics
This compound is orally bioavailable, and its absorption increases when taken with food . It is primarily metabolized by the liver’s CYP3A4 enzymes, but CYP2C8 and CYP2C19 enzymes can also play a role . The half-life of this compound in the plasma is 41 hours in people with HCV . It is excreted mainly through feces (91%) and less than 1% through urine .
Result of Action
The inhibition of the HCV NS3/4A protease by this compound results in the disruption of the viral replication process, thereby preventing the maturation of the virus . This leads to a decrease in the viral load and can result in a sustained virologic response (SVR), which is considered a cure for HCV .
Action Environment
The efficacy of this compound can be influenced by certain polymorphic variants of the virus. Furthermore, the environment within the host’s body, such as the presence of other medications or liver function, can also impact the action and efficacy of this compound .
Wissenschaftliche Forschungsanwendungen
Clinical Efficacy in Hepatitis C Treatment
Simeprevir has demonstrated significant efficacy in treating chronic hepatitis C, particularly in genotype 1 infections. The following key studies highlight its clinical applications:
- PROMISE Study : In this pivotal Phase 3 study, this compound was administered to treatment-experienced patients, resulting in a sustained virologic response (SVR12) rate of 79%, compared to only 37% in the placebo group. This study established this compound's effectiveness when combined with pegylated interferon and ribavirin .
- QUEST Studies : A pooled analysis from the QUEST-1 and QUEST-2 trials showed that 80% of treatment-naïve patients achieved SVR12 when treated with this compound plus pegylated interferon and ribavirin. This was a significant improvement over historical controls .
- This compound Plus Sofosbuvir : A study evaluating this compound combined with sofosbuvir for 12 weeks reported superior SVR12 rates compared to historical controls for both treatment-naïve and treatment-experienced patients .
Patient Subpopulations
This compound's effectiveness varies among different patient demographics and conditions:
- Cirrhosis Patients : In patients with cirrhosis, SVR12 rates were reported at 83% for those treated with this compound plus sofosbuvir. This indicates that even patients with advanced liver disease can benefit significantly from this combination therapy .
- IL28B Genotype Impact : The efficacy of this compound also correlates with the IL28B genotype. In the PROMISE study, SVR12 rates varied significantly based on genotype, highlighting the importance of genetic factors in treatment outcomes .
Safety Profile
This compound is generally well-tolerated, with manageable side effects. The PROMISE study reported on-treatment failure rates of 3% and relapse rates of 19% among patients treated with this compound, which were significantly lower than those observed in the placebo group . Adverse reactions are relatively infrequent and do not exacerbate anemia, a common side effect associated with other treatments like pegylated interferon .
Future Research Directions
Ongoing studies continue to explore the potential applications of this compound beyond its current indications:
- Combination Therapies : Research is being conducted on the use of this compound in combination with newer antiviral agents to enhance efficacy and reduce treatment duration for various HCV genotypes .
- Drug Repositioning : Preliminary investigations suggest that this compound may have applications beyond hepatitis C, including potential effects on other viral infections or conditions due to its mechanism of action targeting viral proteases .
Summary Table of Clinical Trials Involving this compound
| Study Name | Patient Population | Treatment Duration | SVR12 Rate (%) | Key Findings |
|---|---|---|---|---|
| PROMISE | Treatment-experienced | 12 weeks | 79 | Significant improvement over placebo |
| QUEST-1 & QUEST-2 | Treatment-naïve | 12 weeks | 80 | High efficacy in naïve patients |
| OPTIMIST | Patients with cirrhosis | 8-12 weeks | 83 | Effective even in advanced liver disease |
| This compound + Sofosbuvir | Mixed population | 12 weeks | Variable | Superior SVR12 rates compared to historical control |
Biochemische Analyse
Biochemical Properties
Simeprevir plays a crucial role in inhibiting the replication of the hepatitis C virus by targeting the NS3/4A protease, an enzyme essential for the viral life cycle. The compound binds non-covalently to the NS3/4A protease, resulting in a fast association and slow dissociation rate . This interaction prevents the protease from cleaving the HCV polyprotein into functional viral proteins, thereby inhibiting viral replication. This compound also interacts with other biomolecules, such as the cofactor N4A subunit, which is part of the NS3/4A heterodimeric complex .
Cellular Effects
This compound exerts significant effects on various types of cells, particularly hepatocytes, where it accumulates after uptake via organic anion-transporting polypeptides OATP1B1 and OATP1B3 . In hepatocytes, this compound inhibits the NS3/4A protease, leading to a reduction in viral replication. This inhibition affects cell signaling pathways, gene expression, and cellular metabolism by preventing the production of viral proteins necessary for the virus’s life cycle . Additionally, this compound has been shown to display synergistic effects with interferon-α and HCV NS5B inhibitors, further enhancing its antiviral activity .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to the NS3/4A protease, a critical enzyme for the hepatitis C virus. By inhibiting this protease, this compound prevents the cleavage of the HCV polyprotein into individual viral proteins, thereby blocking viral replication . This compound’s binding interactions with the NS3/4A protease are highly specific and potent, making it an effective antiviral agent. Additionally, this compound’s resistance profile differs from first-generation protease inhibitors, as it is less effective against certain polymorphic variants of the NS3 protease .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been observed to change over time. The compound is relatively stable, with a half-life of approximately 41 hours in individuals with HCV . Over time, this compound’s antiviral activity can be influenced by factors such as drug degradation and the development of viral resistance. Long-term studies have shown that this compound maintains its efficacy in reducing viral load and achieving sustained virological response (SVR) when used in combination with other antiviral agents .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. Studies have shown that higher doses of this compound result in more significant reductions in viral replication and improved treatment outcomes . At very high doses, this compound may exhibit toxic or adverse effects, such as hepatotoxicity. It is essential to determine the optimal dosage that maximizes antiviral efficacy while minimizing potential side effects .
Metabolic Pathways
This compound is primarily metabolized in the liver by the cytochrome P450 enzyme CYP3A4, with minor contributions from CYP2C8 and CYP2C19 . The metabolic pathways of this compound involve its conversion into various metabolites, which are then excreted from the body. The compound’s interaction with these enzymes can affect metabolic flux and metabolite levels, influencing its overall efficacy and safety profile .
Transport and Distribution
This compound is transported and distributed within cells and tissues through specific transporters and binding proteins. The compound is taken up into hepatocytes via organic anion-transporting polypeptides OATP1B1 and OATP1B3, where it accumulates and exerts its antiviral effects . This compound’s localization within hepatocytes is crucial for its activity, as it needs to reach the NS3/4A protease to inhibit viral replication effectively .
Subcellular Localization
The subcellular localization of this compound is primarily within the cytoplasm of hepatocytes, where it interacts with the NS3/4A protease . The compound’s activity and function are influenced by its localization, as it needs to be in proximity to the viral protease to exert its inhibitory effects. Post-translational modifications and targeting signals may also play a role in directing this compound to specific compartments within the cell .
Vorbereitungsmethoden
Die Herstellung von TMC435 umfasst verschiedene Synthesewege und Reaktionsbedingungen. Einer der wichtigsten Zwischenprodukte in der Synthese ist ein bicyclisches Lactonamid. Die Herstellungsmethode für dieses Zwischenprodukt umfasst mehrere Schritte, einschließlich der Bildung einer makrocyclischen Verbindung . Die industrielle Produktion von TMC435 beinhaltet typischerweise die Kristallisation des Wirkstoffs, um eine stabile und reine Form zu erhalten, die für die pharmazeutische Verwendung geeignet ist .
Analyse Chemischer Reaktionen
TMC435 unterliegt verschiedenen chemischen Reaktionen, darunter Oxidation, Reduktion und Substitution. Zu den gängigen Reagenzien, die in diesen Reaktionen verwendet werden, gehören organische Lösungsmittel, Säuren und Basen. Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, sind typischerweise Derivate der ursprünglichen Verbindung, die unterschiedliche pharmakologische Eigenschaften haben können .
Vergleich Mit ähnlichen Verbindungen
TMC435 ähnelt anderen Hepatitis-C-Virus-NS3/4A-Protease-Inhibitoren wie Telaprevir und Boceprevir. TMC435 weist jedoch einige einzigartige Merkmale auf, die es von diesen Verbindungen unterscheiden. So weist TMC435 beispielsweise ein günstigeres Resistenzprofil auf und ist gegen ein breiteres Spektrum an Hepatitis-C-Virus-Genotypen wirksam. Darüber hinaus hat sich TMC435 im Vergleich zu anderen Protease-Inhibitoren als sicherer und verträglicher erwiesen .
Ähnliche Verbindungen
- Telaprevir
- Boceprevir
- Grazoprevir
- Paritaprevir
Eigenschaften
CAS-Nummer |
923604-59-5 |
|---|---|
Molekularformel |
C38H47N5O7S2 |
Molekulargewicht |
749.9 g/mol |
IUPAC-Name |
(4S)-N-cyclopropylsulfonyl-17-[7-methoxy-8-methyl-2-(4-propan-2-yl-1,3-thiazol-2-yl)quinolin-4-yl]oxy-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.04,6]octadec-7-ene-4-carboxamide |
InChI |
InChI=1S/C38H47N5O7S2/c1-21(2)30-20-51-35(40-30)29-18-32(26-13-14-31(49-5)22(3)33(26)39-29)50-24-16-27-28(17-24)36(45)43(4)15-9-7-6-8-10-23-19-38(23,41-34(27)44)37(46)42-52(47,48)25-11-12-25/h8,10,13-14,18,20-21,23-25,27-28H,6-7,9,11-12,15-17,19H2,1-5H3,(H,41,44)(H,42,46)/t23?,24?,27?,28?,38-/m0/s1 |
InChI-Schlüssel |
JTZZSQYMACOLNN-ZMKZURCUSA-N |
SMILES |
CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5CC5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC |
Isomerische SMILES |
CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5C[C@@]5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC |
Kanonische SMILES |
CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5CC5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC |
Color/Form |
White to almost white powder |
Piktogramme |
Irritant; Environmental Hazard |
Löslichkeit |
Insoluble Practically insoluble in water over a wide pH range Practically insoluble in propylene glycol; very slightly soluble in ethanol; slightly soluble in acetone. Soluble in dichloromethane; freely soluble in some organic solvents |
Synonyme |
435, TMC 435350, TMC N-(17-(2-(4-isopropylthiazole-2-yl)-7-methoxy-8-methylquinolin-4-yloxy)-13-methyl-2,14-dioxo-3,13-diazatricyclo(13.3.0.04,6)octadec-7-ene-4-carbonyl)(cyclopropyl)sulfonamide Olysio simeprevir TMC 435 TMC 435350 TMC-435 TMC-435350 TMC435 TMC435350 |
Dampfdruck |
5.9X10-27 mm Hg at 25 °C (est) |
Herkunft des Produkts |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.















