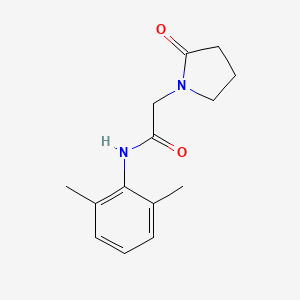
Nefiracetam
Übersicht
Beschreibung
Nefiracetam ist eine nootrope Verbindung, die zur Racetam-Familie gehört. Sie wurde erstmals in den 1990er Jahren in Tokio von Daiichi Pharmaceutical entwickelt. This compound ist bekannt für seine kognitionsfördernden Eigenschaften, darunter Verbesserungen bei Gedächtnis, Stimmung und Motivation. Es ist ein Derivat von Piracetam mit einer ähnlichen chemischen Struktur, jedoch mit einer Phenylgruppe und zwei Methylgruppen, die an das Amin addiert werden, wodurch seine Potenz im Vergleich zu anderen Racetamen erhöht wird .
2. Herstellungsmethoden
Synthesewege und Reaktionsbedingungen: this compound kann durch einen mehrstufigen Prozess synthetisiert werden, der die Reaktion von 2,6-Dimethylphenylamin mit Ethylchloracetat beinhaltet, um ein Zwischenprodukt zu bilden, das dann cyclisiert wird, um this compound zu erzeugen. Die Reaktionsbedingungen umfassen typischerweise die Verwendung von Lösungsmitteln wie Ethanol und Katalysatoren wie Natriumethoxid .
Industrielle Produktionsmethoden: Die industrielle Produktion von this compound beinhaltet ähnliche Synthesewege, jedoch in größerem Maßstab. Der Prozess ist für höhere Ausbeuten und Reinheit optimiert und beinhaltet oft zusätzliche Reinigungsschritte wie Umkristallisation und Chromatographie, um sicherzustellen, dass das Endprodukt den pharmazeutischen Standards entspricht .
Wissenschaftliche Forschungsanwendungen
Pharmacological Mechanisms
Nefiracetam exhibits several pharmacological actions that contribute to its nootropic effects:
- Cholinergic System Modulation : this compound enhances the activity of nicotinic acetylcholine receptors, which are crucial for cognitive functions such as learning and memory. It interacts with a protein kinase C pathway, promoting acetylcholine turnover and release .
- Glutamatergic System Interaction : Research indicates that this compound potentiates N-methyl-D-aspartate (NMDA) receptor currents, enhancing synaptic transmission and potentially improving cognitive function .
- GABAergic Activity : this compound shows high affinity for GABA A receptors, suggesting it may function as an agonist, which could help mitigate anxiety-related symptoms .
Alzheimer's Disease
This compound has been investigated for its potential in treating Alzheimer's disease. A double-blind, placebo-controlled trial aims to assess its efficacy in improving cognitive function among patients with mild to moderate dementia. The study utilizes standardized neuropsychological tests to evaluate outcomes .
Post-Stroke Rehabilitation
This compound has been evaluated for its effects on post-stroke apathy. A randomized controlled trial involving 13 patients found no significant difference between this compound and placebo in reducing apathy scores . Despite this, there is ongoing interest in its potential benefits for cognitive recovery post-stroke .
Antiepileptic Potential
In animal models, this compound has demonstrated anticonvulsant properties by reducing the severity and duration of seizures induced by amygdala kindling. This suggests a potential application in epilepsy management .
Case Studies and Research Findings
| Study Title | Sample Size | Findings | |
|---|---|---|---|
| Efficacy of this compound in Alzheimer's Disease | TBD | Evaluated cognitive improvement through neuropsychological tests | Ongoing study; results pending |
| This compound for Post-Stroke Apathy | 13 | No significant difference compared to placebo | Limited efficacy; highlights need for larger trials |
| Anticonvulsant Effects in Rats | TBD | Reduced seizure severity and duration in kindled rats | Suggests potential for epilepsy treatment |
Wirkmechanismus
Target of Action
Nefiracetam is a nootropic drug that primarily targets neurotransmitter systems in the brain, including the GABAergic, cholinergic, and monoaminergic neuronal systems . It also shows high affinity for the GABA A receptor .
Mode of Action
This compound modulates neurotransmission by influencing calcium channels and acetylcholine receptors . It enhances both GABAergic and cholinergic signalling . It’s also known to interact with nicotinic acetylcholine receptors , which play a crucial role in cognitive processes . This compound is presumed to be an agonist at the GABA A receptor .
Biochemical Pathways
This compound’s cytoprotective actions are mediated by enhancement of GABAergic, cholinergic, and monoaminergic neuronal systems . It modulates the GABAA receptor-channel, playing a significant role in reducing neuronal excitability throughout the nervous system . It also interacts with nicotinic acetylcholine receptors, crucial for cognitive processes .
Pharmacokinetics
It’s known that this compound is administered orally and has an elimination half-life of 3-5 hours .
Result of Action
This compound’s action results in a variety of molecular and cellular effects. It’s known for its cholinergic and gabaergic actions and modulates neurotransmission . It’s also known to enhance cognitive performance and information processing speed . Long-term usage is both neuroprotective and nootropic in research animals .
Action Environment
This suggests that improved memory-associated synaptic plasticity may be the fundamental mechanism underlying the disease-modifying action of drugs such as this compound .
Biochemische Analyse
Biochemical Properties
Nefiracetam interacts with various enzymes, proteins, and other biomolecules. It shows high affinity for the GABA A receptor, where it is presumed to be an agonist . It enhances both GABAergic and cholinergic signalling . It also exhibits antiamnesic effects against a number of memory impairing substances .
Cellular Effects
This compound has significant effects on various types of cells and cellular processes. It influences cell function by enhancing signalling of acetylcholine and glutamate at the synapse and then prolonging the calcium in the activated neuron . It also modulates the GABAA receptor-channel, playing a significant role in reducing neuronal excitability throughout the nervous system .
Molecular Mechanism
This compound exerts its effects at the molecular level through several mechanisms. It prolongs the opening of calcium channels, which enhances signalling of a receptor independent of the synapse . It also augments signalling through cholinergic receptors, which then releases most excitatory neurotransmitters from the presynaptic level .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound change over time. It does not appear to significantly affect memory formation acutely, but it can increase memory formation when taken daily over a prolonged period of time . It also shows a higher rate of neurogenesis with prolonged supplementation .
Dosage Effects in Animal Models
In animal models, the effects of this compound vary with different dosages. Animal studies using acute doses tend to note most benefits in the 3-10mg/kg range . This range has been repeatedly shown to enhance memory formation when taken daily over a prolonged period of time .
Metabolic Pathways
This compound is involved in various metabolic pathways. It is extensively metabolised, and its major metabolites in humans are 5-hydroxy-nefiracetam, 4-hydroxy-nefiracetam and N-[(2,6-dimethylphenylcarbamoyl)methyl]-succinamic acid .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Nefiracetam can be synthesized through a multi-step process involving the reaction of 2,6-dimethylphenylamine with ethyl chloroacetate to form an intermediate, which is then cyclized to produce this compound. The reaction conditions typically involve the use of solvents such as ethanol and catalysts like sodium ethoxide .
Industrial Production Methods: Industrial production of this compound involves similar synthetic routes but on a larger scale. The process is optimized for higher yields and purity, often involving additional purification steps such as recrystallization and chromatography to ensure the final product meets pharmaceutical standards .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Nefiracetam unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: this compound kann oxidiert werden, um hydroxylierte Derivate zu bilden.
Reduktion: Reduktionsreaktionen können this compound in sein entsprechendes Amin umwandeln.
Substitution: this compound kann nukleophile Substitutionsreaktionen eingehen, bei denen die Aminogruppe durch andere Nukleophile ersetzt wird.
Häufige Reagenzien und Bedingungen:
Oxidation: Häufige Oxidationsmittel umfassen Kaliumpermanganat und Wasserstoffperoxid.
Reduktion: Reduktionsmittel wie Lithiumaluminiumhydrid und Natriumborhydrid werden verwendet.
Substitution: Nukleophile wie Natriumazid und Thiole werden häufig eingesetzt.
Wichtigste gebildete Produkte:
Oxidation: Hydroxylierte this compound-Derivate.
Reduktion: Aminierte this compound-Derivate.
Substitution: Verschiedene substituierte this compound-Verbindungen, abhängig von dem verwendeten Nukleophil.
Vergleich Mit ähnlichen Verbindungen
Nefiracetam wird mit anderen Racetamen verglichen, wie z. B.:
Piracetam: Das erste Racetam, bekannt für seine kognitionsfördernden Wirkungen, aber weniger potent als this compound.
Aniracetam: Ähnlich wie this compound, aber mit zusätzlichen anxiolytischen Eigenschaften.
Oxiracetam: Bekannt für seine anregenden Wirkungen und kognitionsfördernden Eigenschaften.
Phenylpiracetam: Potenter als Piracetam, mit zusätzlichen stimulierenden Eigenschaften.
Pramiracetam: Hochpotent, hauptsächlich zur Verbesserung des Gedächtnisses eingesetzt
This compound zeichnet sich durch seine einzigartige Kombination aus kognitionsfördernder Wirkung, Neuroprotection und Stimmungsverbesserung aus, was es zu einer vielseitigen Verbindung in der Racetam-Familie macht.
Biologische Aktivität
Nefiracetam, a pyrrolidone-type nootropic compound, has garnered attention for its potential cognitive-enhancing properties and various pharmacological effects. This article delves into the biological activity of this compound, highlighting its mechanisms of action, efficacy in clinical studies, and relevant case studies.
This compound exhibits multiple mechanisms that contribute to its biological activity:
- NMDA Receptor Modulation : this compound has been shown to potentiate the activity of N-methyl-D-aspartate (NMDA) receptors. It enhances NMDA currents through an allosteric mechanism, interacting with protein kinase C (PKC) pathways, which leads to increased phosphorylation and modulation of glycine binding sites on NMDA receptors. This effect is particularly significant as NMDA receptors play a crucial role in synaptic plasticity and memory formation .
- Cholinergic System Activation : The compound activates cholinergic pathways, which are essential for memory and learning. It has been observed to increase acetylcholine levels in the brain, thereby enhancing cognitive functions .
- Calcium Channel Activation : this compound activates L/N-type calcium channels, facilitating calcium influx that is critical for neurotransmitter release and neuronal excitability .
- Neuroprotective Effects : In models of retinal ischemia-reperfusion injury, this compound demonstrated significant neuroprotective actions, suggesting its potential utility in conditions involving oxidative stress and neuronal damage .
Case Studies and Clinical Trials
- Poststroke Apathy Study : A randomized, placebo-controlled trial evaluated the efficacy of this compound in treating apathy in stroke patients. Patients receiving 900 mg/day showed a decrease in apathy scores; however, the results did not reach statistical significance compared to placebo due to a small sample size (only 20 patients with apathy were randomized) . This highlights the need for larger multicenter studies to validate these findings.
- Cognitive Enhancement in Older Rabbits : Research conducted on older rabbits indicated that this compound significantly improved retention and relearning capabilities. The study found lasting effects on memory performance even after treatment cessation, suggesting a prolonged impact on cognitive functions .
- GABAergic Activity : In an animal model of cerebral ischemia, this compound was shown to restore GABA levels and enhance GABAergic activity, indicating its role in modulating inhibitory neurotransmission during ischemic events. This effect was more pronounced than its influence on cholinergic systems .
Summary of Findings
| Study Type | Sample Size | Dosage | Outcome |
|---|---|---|---|
| Poststroke Apathy | 20 | 900 mg/day | Decreased apathy scores; not statistically significant |
| Cognitive Enhancement (Rabbits) | 34 | 5-15 mg/kg | Improved retention and relearning capabilities |
| GABAergic Activity | Rat Model | 10 mg/kg | Restored GABA levels post-ischemia |
Eigenschaften
IUPAC Name |
N-(2,6-dimethylphenyl)-2-(2-oxopyrrolidin-1-yl)acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H18N2O2/c1-10-5-3-6-11(2)14(10)15-12(17)9-16-8-4-7-13(16)18/h3,5-6H,4,7-9H2,1-2H3,(H,15,17) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NGHTXZCKLWZPGK-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C(=CC=C1)C)NC(=O)CN2CCCC2=O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H18N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2020923 | |
| Record name | Nefiracetam | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2020923 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
246.30 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
77191-36-7 | |
| Record name | Nefiracetam | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=77191-36-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nefiracetam [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0077191367 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nefiracetam | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB13082 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nefiracetam | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759830 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Nefiracetam | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2020923 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | N-(2,6-dimethylphenyl)-2-(2-oxopyrrolidin-1-yl)acetamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | NEFIRACETAM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1JK12GX30N | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details







Synthesis routes and methods III
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.













