3QKI37EEHE
Übersicht
Beschreibung
Remdesivir wurde ursprünglich von Gilead Sciences zur Behandlung der Ebola-Viruskrankheit entwickelt und hat seitdem große Aufmerksamkeit für seinen potenziellen Einsatz gegen andere Virusinfektionen, einschließlich des schweren akuten respiratorischen Syndroms Coronavirus 2 (SARS-CoV-2), dem Virus, das für COVID-19 verantwortlich ist .
Herstellungsmethoden
Synthesewege und Reaktionsbedingungen
Die Synthese von Remdesivir umfasst mehrere Schritte, beginnend mit kommerziell erhältlichen Ausgangsmaterialien. Die wichtigsten Schritte umfassen die Bildung des Nukleosid-Analogons und dessen anschließende Phosphorylierung, um die aktive Triphosphatform zu erzeugen. Der Syntheseweg beinhaltet typischerweise Schutz- und Entschützungsschritte, nukleophile Substitutionsreaktionen und Phosphorylierungsreaktionen unter kontrollierten Bedingungen .
Industrielle Produktionsmethoden
Die industrielle Produktion von Remdesivir folgt einem ähnlichen Syntheseweg, ist aber für die großtechnische Herstellung optimiert. Dies beinhaltet die Verwendung von Reaktionen mit hoher Ausbeute, effizienten Reinigungsmethoden und strengen Qualitätskontrollmaßnahmen, um die Konsistenz und Reinheit des Endprodukts zu gewährleisten .
Wissenschaftliche Forschungsanwendungen
Efficacy in Clinical Trials
Numerous clinical trials have evaluated the efficacy of Remdesivir in treating COVID-19. Key findings from several studies are summarized below:
Safety Profile
The safety profile of Remdesivir has been evaluated across multiple studies. Common adverse effects include hepatic impairment; however, the incidence of adverse events was similar between treated and control groups . Ongoing studies continue to monitor long-term safety and efficacy.
Case Study: Efficacy in Severe COVID-19
A systematic review involving over 40 clinical trials indicated that Remdesivir might significantly shorten recovery time among hospitalized adults with severe COVID-19. Observational studies reported reduced mortality rates associated with Remdesivir treatment compared to standard care .
Observational Study: Impact on Mortality Rates
A large-scale observational study involving over 121,000 patients found that Remdesivir-treated individuals had a lower mortality rate within 14 and 28 days compared to those not receiving the drug. The adjusted hazard ratios indicated a statistically significant reduction in mortality associated with Remdesivir use .
Regulatory Approvals and Recommendations
Remdesivir received Emergency Use Authorization from the U.S. Food and Drug Administration for treating COVID-19 in hospitalized patients. The National Institute of Allergy and Infectious Diseases has also endorsed its use based on clinical trial data demonstrating its benefits .
Wirkmechanismus
Target of Action
Remdesivir, also known as GS-5734, is a nucleoside analog . Its primary target is the RNA-dependent RNA polymerase (RdRp) enzyme complex of RNA viruses . This enzyme complex is crucial for the replication of the viral genome .
Mode of Action
Remdesivir acts by inhibiting the replication of the virus . It is metabolically activated to form an intracellular active triphosphate metabolite, GS-443902 . This active metabolite is incorporated into the viral RNA chains, thereby inhibiting the RdRp and preventing the virus from growing and spreading in the body . It does this by terminating RNA transcription prematurely .
Biochemical Pathways
Remdesivir affects the biochemical pathway of viral replication. By inhibiting the RdRp enzyme complex, it disrupts the replication of the viral RNA genome . This results in the termination of the viral RNA chains, thereby preventing the virus from replicating and spreading within the host organism .
Pharmacokinetics
Remdesivir exhibits linear pharmacokinetics following single-dose intravenous administration . It undergoes metabolic activation to form the intracellular active triphosphate metabolite, GS-443902 . The major metabolite GS-441524 accumulates approximately 1.9-fold in plasma following multiple doses of Remdesivir 150 mg once daily for 7 or 14 days . The clinical dose regimen of a 200-mg loading dose on day 1 followed by 100-mg maintenance doses for 4 or 9 days was selected for further evaluation of pharmacokinetics and safety .
Result of Action
The result of Remdesivir’s action is the inhibition of viral replication. This can help the body’s immune system effectively control the infection by stopping the virus from growing and spreading in the body . It has demonstrated in vitro and in vivo activity against SARS-CoV-2 .
Action Environment
The efficacy of Remdesivir can be influenced by various environmental factors. For instance, the drug’s effectiveness may vary depending on the patient’s health status and the presence of other diseases . Additionally, the drug’s pharmacokinetics can be affected by factors such as the patient’s age, weight, and renal function .
Biochemische Analyse
Biochemical Properties
Remdesivir interacts with the viral RNA-dependent RNA polymerase, an enzyme crucial for viral replication . It is metabolized by the host cell into its active form, a triphosphate (TP) metabolite . This metabolite inhibits viral replication by acting as a chain terminator during the synthesis of viral RNA .
Cellular Effects
Remdesivir has been shown to inhibit the replication of various coronaviruses in human airway epithelial cells . It reduces viral load and improves clinical signs of disease as well as respiratory function .
Molecular Mechanism
The active triphosphate metabolite of Remdesivir incorporates into the growing RNA chain during viral replication. This causes premature termination of the RNA synthesis, thereby inhibiting the replication of the virus . It’s worth noting that Remdesivir can exert its effects even in the presence of the viral proofreading exoribonuclease, an enzyme that often complicates the development of antiviral nucleosides .
Temporal Effects in Laboratory Settings
In laboratory settings, Remdesivir has shown to reduce viral RNA levels in a dose-dependent manner that parallels impairment of viral titer . While it is highly active against wild-type viruses, it has been found to be even more active against viruses lacking the proofreading activity of the exoribonuclease .
Dosage Effects in Animal Models
In animal models, both prophylactic and early therapeutic administration of Remdesivir have significantly reduced lung viral load and improved clinical signs of disease . A study on male mice suggested that a high dosage of Remdesivir may induce testicular toxicity and result in deterioration of sperm parameters .
Metabolic Pathways
Remdesivir is a prodrug that requires metabolism by the host cell to form the pharmacologically active triphosphate metabolite
Transport and Distribution
As a prodrug, it is designed to deliver the nucleoside monophosphate into the cell, thereby circumventing the rate-limiting first phosphorylation step and allowing for efficient intracellular delivery .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions
The synthesis of GS-5734 involves multiple steps, starting from commercially available starting materials. The key steps include the formation of the nucleoside analog and its subsequent phosphorylation to produce the active triphosphate form. The synthetic route typically involves protection and deprotection steps, nucleophilic substitution reactions, and phosphorylation reactions under controlled conditions .
Industrial Production Methods
Industrial production of GS-5734 follows a similar synthetic route but is optimized for large-scale manufacturing. This involves the use of high-yielding reactions, efficient purification methods, and stringent quality control measures to ensure the consistency and purity of the final product .
Analyse Chemischer Reaktionen
Arten von Reaktionen
Remdesivir durchläuft verschiedene Arten von chemischen Reaktionen, darunter:
Oxidation: Remdesivir kann oxidiert werden, um verschiedene Metaboliten zu bilden.
Reduktion: Reduktionsreaktionen können die Struktur des Nukleosid-Analogons verändern.
Substitution: Nukleophile Substitutionsreaktionen sind an der Synthese von Remdesivir beteiligt
Häufige Reagenzien und Bedingungen
Häufige Reagenzien, die bei der Synthese und Reaktion von Remdesivir verwendet werden, umfassen:
Phosphorylierungsmittel: Verwendet für die Phosphorylierungsschritte.
Schutzgruppen: Verwendet, um funktionelle Gruppen während der Synthese zu schützen.
Lösungsmittel: Wie Dimethylsulfoxid (DMSO) und Acetonitril, die als Reaktionsmedium verwendet werden.
Hauptprodukte, die gebildet werden
Die Hauptprodukte, die aus den Reaktionen von Remdesivir gebildet werden, umfassen seine aktive Triphosphatform, Remdesivir-Triphosphat, und verschiedene Metaboliten, die durch Oxidations- und Reduktionsreaktionen gebildet werden .
Vergleich Mit ähnlichen Verbindungen
Ähnliche Verbindungen
GS-441524: Ein parentales Nukleosid-Analogon von Remdesivir mit ähnlicher antiviraler Aktivität.
Favipiravir: Ein weiteres Nukleosid-Analogon mit breitspektrigem antiviralen Aktivität.
Ribavirin: Ein Nukleosid-Analogon, das zur Behandlung verschiedener Virusinfektionen eingesetzt wird.
Einzigartigkeit von Remdesivir
Remdesivir ist einzigartig aufgrund seiner breitspektrigen antiviralen Aktivität und seiner Fähigkeit, eine große Bandbreite an RNA-Viren, einschließlich Coronaviren und Filoviren, zu hemmen. Seine Wirksamkeit gegen SARS-CoV-2 hat es zu einem wichtigen Bestandteil im Kampf gegen COVID-19 gemacht .
Biologische Aktivität
Remdesivir, a nucleotide analog prodrug, has garnered significant attention as a therapeutic agent against viral infections, particularly COVID-19. This article delves into the biological activity of remdesivir, focusing on its mechanism of action, pharmacokinetics, clinical efficacy, and ongoing research.
Remdesivir (GS-5734) is designed to inhibit viral RNA-dependent RNA polymerase (RdRp), crucial for the replication of RNA viruses. Upon entering host cells, remdesivir is metabolized into its active form, GS-443902, which mimics adenosine triphosphate (ATP). This active form competes with ATP for incorporation into the viral RNA strand during replication. The incorporation of GS-443902 leads to premature termination of RNA synthesis, thereby limiting viral replication.
Key Mechanisms:
- Nucleotide Analog: Resembles ATP and integrates into viral RNA.
- Chain Termination: Causes premature termination of RNA synthesis.
- Broad Spectrum Activity: Effective against various coronaviruses (SARS-CoV, MERS-CoV) and other RNA viruses like Ebola .
Pharmacokinetics
The pharmacokinetics of remdesivir have been studied primarily through intravenous administration. A randomized controlled trial indicated that remdesivir exhibits linear pharmacokinetics across various doses (3 to 225 mg). Notably, it is a substrate for organic anion transporting polypeptides (OATP1B1 and OATP1B3), influencing its hepatic uptake and elimination .
Table 1: Pharmacokinetic Profile of Remdesivir
| Parameter | Value |
|---|---|
| Bioavailability | Not applicable (IV only) |
| Half-life | Approximately 1 hour |
| Volume of distribution | 1 L/kg |
| Clearance | 0.5 L/h/kg |
Clinical Efficacy
Several clinical trials have evaluated the efficacy of remdesivir in treating COVID-19. The most notable studies include:
- ACTT-1 Trial: A randomized trial demonstrating that patients receiving remdesivir had a shorter time to recovery compared to those receiving standard care.
- SIMPLE Trials: These trials assessed the efficacy of 5-day versus 10-day treatment regimens in hospitalized patients with severe COVID-19. Results indicated that both regimens provided similar clinical improvements .
Table 2: Summary of Key Clinical Trials
Case Studies and Observational Data
Observational studies have also provided insights into the efficacy and safety profile of remdesivir. For instance, a study involving hospitalized patients showed that those treated with a 5-day course had statistically significant improvements in clinical status at Day 11 compared to those receiving standard care .
Case Study Example:
A cohort study analyzed outcomes in critically ill patients treated with remdesivir. It found that early administration correlated with improved survival rates and reduced need for mechanical ventilation.
Ongoing Research and Future Directions
Research continues to explore the full potential of remdesivir beyond COVID-19. Current studies are investigating:
- Combination therapies with other antiviral agents.
- Efficacy in pediatric populations.
- Alternative formulations to enhance delivery methods.
Eigenschaften
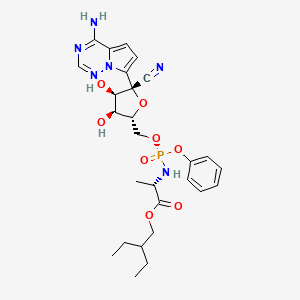
IUPAC Name |
2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H35N6O8P/c1-4-18(5-2)13-38-26(36)17(3)32-42(37,41-19-9-7-6-8-10-19)39-14-21-23(34)24(35)27(15-28,40-21)22-12-11-20-25(29)30-16-31-33(20)22/h6-12,16-18,21,23-24,34-35H,4-5,13-14H2,1-3H3,(H,32,37)(H2,29,30,31)/t17-,21+,23+,24+,27-,42-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RWWYLEGWBNMMLJ-YSOARWBDSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC(CC)COC(=O)C(C)NP(=O)(OCC1C(C(C(O1)(C#N)C2=CC=C3N2N=CN=C3N)O)O)OC4=CC=CC=C4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCC(CC)COC(=O)[C@H](C)N[P@](=O)(OC[C@@H]1[C@H]([C@H]([C@](O1)(C#N)C2=CC=C3N2N=CN=C3N)O)O)OC4=CC=CC=C4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H35N6O8P | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID701022537 | |
| Record name | Remdesivir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701022537 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
602.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
COVID-19 is caused by the positive-sense RNA virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Replication of the viral genome is a key step in the infectious cycle of RNA viruses, including those of the _Filoviridae_, _Paramyxoviridae_, _Pneumoviridae_, and _Coronaviridae_ families, and is carried out by viral RNA-dependent RNA polymerase (RdRp) enzymes or enzyme complexes. For both SARS-CoV and SARS-CoV-2, the RdRp comprises nsp7, nsp8, and nsp12 subunits under physiological conditions, although functional RdRp complexes can be reassembled _in vitro_ that incorporate only the nsp8 and nsp12 subunits, similar to the Middle East respiratory syndrome coronavirus (MERS-CoV). Remdesivir is a phosphoramidite prodrug of a 1'-cyano-substituted adenosine nucleotide analogue that competes with ATP for incorporation into newly synthesized viral RNA by the corresponding RdRp complex. Remdesivir enters cells before being cleaved to its monophosphate form through the action of either carboxylesterase 1 or cathepsin A; it is subsequently phosphorylated by undescribed kinases to yield its active triphosphate form remdesivir triphosphate (RDV-TP or GS-443902). RDV-TP is efficiently incorporated by the SARS-CoV-2 RdRp complex, with a 3.65-fold selectivity for RDV-TP over endogenous ATP. Unlike some nucleoside analogues, remdesivir provides a free 3'-hydroxyl group that allows for continued chain elongation. However, modelling and _in vitro_ experiments suggest that at _i_ + 4 (corresponding to the position for the incorporation of the fourth nucleotide following RDV-TP incorporation), the 1'-cyano group of remdesivir sterically clashes with Ser-861 of the RdRp, preventing further enzyme translocation and terminating replication at position _i_ + 3. This mechanism was essentially identical between SARS-CoV, SARS-CoV-2, and MERS-CoV, and genomic comparisons reveal that Ser-861 is conserved across alpha-, beta-, and deltacoronaviruses, suggesting remdesivir may possess broad antiviral activity. Considerations for the use of nucleotide analogues like remdesivir include the possible accumulation of resistance mutations. Excision of analogues through the 3'-5' exonuclease (ExoN) activity of replication complexes, mediated in SARS-CoV by the nsp14 subunit, is of possible concern. Murine hepatitis viruses (MHVs) engineered to lack ExoN activity are approximately 4-fold more susceptible to remdesivir, supporting the proposed mechanism of action. However, the relatively mild benefit of ExoN activity to remdesivir resistance is proposed to involve its delayed chain termination mechanism, whereby additional endogenous nucleotides are incorporated following RDV-TP. In addition, serial passage of MHV in increasing concentrations of the remdesivir parent molecule [GS-441524] led to the development of resistance mutations F476L and V553L, which maintain activity when transferred to SARS-CoV. However, these mutant viruses are less fit than wild-type in both competition assays and _in vivo_ in the absence of selective pressure. To date, no clinical data on SARS-CoV-2 resistance to remdesivir have been described. | |
| Record name | Remdesivir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14761 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1809249-37-3 | |
| Record name | L-Alanine, N-[(S)-hydroxyphenoxyphosphinyl]-, 2-ethylbutyl ester, 6-ester with 2-C-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2,5-anhydro-D-altrononitrile | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1809249-37-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Remdesivir [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1809249373 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Remdesivir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14761 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Remdesivir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701022537 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | REMDESIVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3QKI37EEHE | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.















