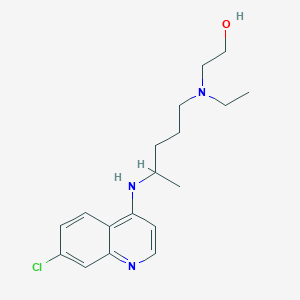
Hydroxychloroquin
Übersicht
Beschreibung
Hydroxychloroquin ist ein Medikament, das hauptsächlich zur Vorbeugung und Behandlung von Malaria in Gebieten eingesetzt wird, in denen Malaria noch auf Chloroquin anspricht. Es wird auch zur Behandlung von rheumatoider Arthritis, Lupus und Porphyria cutanea tarda verwendet. This compound wird oral eingenommen, häufig in Form von this compound-Sulfat . Es gehört zu den Medikamentenfamilien der Antimalariamittel und 4-Aminoquinoline .
Wissenschaftliche Forschungsanwendungen
Antimalarial Use
Primary Indication:
- Hydroxychloroquine is primarily indicated for the treatment and prophylaxis of malaria, particularly in regions where chloroquine resistance is not prevalent. It acts by inhibiting the growth of the malaria parasite within red blood cells.
Autoimmune Diseases
Rheumatological Applications:
- Systemic Lupus Erythematosus (SLE): HCQ is widely used in managing SLE due to its immunomodulatory effects. It helps reduce disease flares and improves overall prognosis by decreasing inflammation and preventing thrombotic events associated with antiphospholipid syndrome .
- Rheumatoid Arthritis (RA): HCQ serves as a disease-modifying antirheumatic drug (DMARD) that alleviates symptoms and slows disease progression in RA patients .
Infectious Diseases
COVID-19 Research:
- During the COVID-19 pandemic, hydroxychloroquine garnered attention for its potential antiviral properties against SARS-CoV-2. Several studies investigated its efficacy in critically ill patients:
- A retrospective study indicated that HCQ treatment was associated with decreased mortality rates and reduced inflammatory cytokine levels in critically ill COVID-19 patients .
- A systematic review highlighted mixed results regarding HCQ's effectiveness in treating COVID-19, emphasizing the need for larger, well-designed clinical trials to confirm its therapeutic role .
Emerging Applications
Other Potential Uses:
- Research is ongoing into HCQ's efficacy against various viral infections, including HIV, Zika virus, and chikungunya . Its immunomodulatory properties also suggest potential applications in treating other autoimmune conditions beyond SLE and RA.
Data Table: Summary of Hydroxychloroquine Applications
Case Studies
- COVID-19 Treatment Study:
- Lupus Management:
Wirkmechanismus
Target of Action
Hydroxychloroquine (HCQ) is an antimalarial medication that also has immunomodulatory properties, making it useful for treating autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus . The primary targets of HCQ are Toll-like receptors (TLRs) , which exist on the surface of endosomes and play a significant role in the innate immune response and in autoimmune disease . HCQ may also target autophagy-related proteins such as ribosyldihydronicotinamide dehydrogenase (NQO2) and transport protein Sec23A (SEC23A) .
Mode of Action
HCQ exerts its effects by interacting with its targets and causing changes in cellular processes. It inhibits the activation of TLRs through two mechanisms: by increasing lysosomal pH, preventing proteolytic cleavage required for TLR activation, and/or by directly binding to DNA and RNA, thus preventing their interaction with TLR receptors in endosomes . Furthermore, HCQ may exert its functions by targeting autophagy-related proteins or regulating the expression of certain proteins .
Biochemical Pathways
HCQ affects several biochemical pathways. It is known to interfere with the endocytic pathway, blockade of sialic acid receptors, restriction of pH mediated spike (S) protein cleavage at the angiotensin-converting enzyme 2 (ACE2) binding site, and prevention of cytokine storm . It also affects the autophagosome–lysosomal pathway and sphingolipid metabolism .
Pharmacokinetics
HCQ is a weak base and has a characteristic ‘deep’ volume of distribution and a half-life of around 50 days . It is metabolized in the liver by cytochrome P450 (CYP) enzymes into two metabolites, desethylchloroquine and bisdesethylchloroquine . About 60% of both the drugs remain unchanged and about 40% of the drugs are metabolized . HCQ is also known to interfere with other drugs as it is a substrate for CYP enzymes .
Result of Action
The molecular and cellular effects of HCQ’s action include the reduction of inflammatory molecules and improvement in the clearance of Alzheimer’s-related beta-amyloid protein and abnormal tau accumulation . It also results in loss of inner retinal neurons and retinal ganglion cells (RGC) and compromises visual functions .
Action Environment
Environmental factors can influence the action, efficacy, and stability of HCQ. It has been found that HCQ is ecologically persistent and bioaccumulative, intensifying its presence in different environmental matrices due to improper treatment of waste and no proper legislation . Administration and excretion of HCQ, along with its toxic metabolites, contaminate surface and groundwater . This raises long-term toxic concerns from cellular biochemical changes to mortality in target and non-target organisms .
Biochemische Analyse
Biochemical Properties
Hydroxychloroquine is a weak base that accumulates in acidic compartments such as lysosomes and inflamed tissues . It interferes with lysosomal activity and autophagy, interacts with membrane stability, and alters signaling pathways and transcriptional activity . This can result in inhibition of cytokine production and modulation of certain co-stimulatory molecules .
Cellular Effects
Hydroxychloroquine has been shown to have a variety of effects on cells. It can inhibit terminal glycosylation of ACE2, the receptor that SARS-CoV and SARS-CoV-2 target for cell entry . ACE2 that is not in the glycosylated state may less efficiently interact with the SARS-CoV-2 spike protein, further inhibiting viral entry . Hydroxychloroquine also acts by suppressing Toll-like receptors to trigger important immunomodulatory effects .
Temporal Effects in Laboratory Settings
In laboratory settings, severe laboratory abnormalities while taking hydroxychloroquine are rare, even in a population with a high rate of comorbidities . Among the abnormalities observed, the majority of them were likely due to disease progression or a medication other than hydroxychloroquine .
Dosage Effects in Animal Models
In animal models, hydroxychloroquine has been shown to be ineffective in preventing or treating SARS-CoV-2 infection, regardless of the dosage used . The LD50 (lethal dose, 50%) of hydroxychloroquine is approximately twice as high as that of chloroquine .
Metabolic Pathways
Hydroxychloroquine is metabolized by CYP3A4, CYP2D6, and CYP2C8 in vitro . All three CYPs formed the primary metabolites desethylchloroquine (DCQ) and desethylhydroxychloroquine (DHCQ) to various degrees .
Transport and Distribution
Hydroxychloroquine is completely absorbed from the gastrointestinal tract, sequestered in peripheral tissues, metabolized in the liver to pharmacologically active by-products, and excreted via the kidneys and the feces . Plasma volumes of distribution up to 65,000 L for chloroquine and 44,257 L for hydroxychloroquine have been reported .
Subcellular Localization
Hydroxychloroquine and its metabolites are primarily localized in the cytoplasm . In some cell lines, they accumulate in a specific region of the cytoplasm .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen: Die Herstellung von Hydroxychloroquin umfasst mehrere Schritte. Ein Verfahren beinhaltet den Schutz der Hydroxylgruppe von 5-(N-Ethyl-N-Hydroxyethyl)-2-Aminopentan unter Verwendung eines Silanierungsreagenzes. Die Aminoprotonen werden dann in Tetrahydrofuran oder Toluol unter Verwendung einer Bis(trimethylsilyl)lithium-Amid-Lösung entfernt, um Aminoanionen zu bilden. Diese Anionen unterliegen einer Substitutionsreaktion mit 4,7-Dichlorchinolin, um this compound zu erzeugen . Das this compound-Sulfat wird dann mit Schwefelsäure in einer alkoholischen Lösung verestert, um this compound-Sulfat zu erzeugen .
Industrielle Produktionsverfahren: Industrielle Produktionsverfahren für this compound-Sulfat beinhalten die Kondensation von 4,7-Dichlorchinolin mit einer this compound-Seitenkette unter Einwirkung eines Katalysators, um this compound zu erhalten. Anschließend wird this compound mit Schwefelsäure umgesetzt, um this compound-Sulfat herzustellen .
Analyse Chemischer Reaktionen
Reaktionstypen: Hydroxychloroquin unterliegt verschiedenen chemischen Reaktionen, darunter Oxidations-, Reduktions- und Substitutionsreaktionen.
Häufige Reagenzien und Bedingungen: Häufig verwendete Reagenzien in diesen Reaktionen sind Bis(trimethylsilyl)lithium-Amid zur Entfernung von Aminoprotonen und Schwefelsäure für den Veresterungsprozess .
Hauptprodukte, die gebildet werden: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, sind this compound und this compound-Sulfat .
Vergleich Mit ähnlichen Verbindungen
Hydroxychloroquin ähnelt Chloroquin, beides sind 4-Aminoquinolin-Verbindungen, die zur Behandlung von Malaria und Autoimmunerkrankungen eingesetzt werden . This compound hat eine geringere Häufigkeit von potenziell irreversiblen okulären/retinalen Toxizitäten im Vergleich zu Chloroquin . Andere ähnliche Verbindungen sind Desethylthis compound und Oxichlorochin .
Biologische Aktivität
Hydroxychloroquine (HCQ) is a medication traditionally used for treating malaria and autoimmune diseases such as lupus and rheumatoid arthritis. Its biological activity has garnered significant attention, particularly in the context of viral infections, including COVID-19. This article explores the diverse mechanisms through which HCQ exerts its effects, supported by research findings, data tables, and case studies.
HCQ operates through several biological pathways, which can be categorized into five major mechanisms:
- Alkalinization of Lysosomes and Endosomes : HCQ increases the pH within lysosomes and endosomes, inhibiting the processing of immune complexes and viral particles. This alkalinization disrupts critical cellular pathways necessary for viral replication .
- Inhibition of TLR7 and TLR9 : HCQ accumulates in endosomal compartments, inhibiting Toll-like receptors (TLR7 and TLR9) that play a crucial role in the immune response. By preventing these receptors from binding to immune complexes, HCQ reduces the transcription of type-1 interferons, leading to immunomodulatory effects .
- Downregulation of CXCR4 Expression : HCQ has been shown to downregulate C-X-C chemokine receptor type 4 (CXCR4), which is involved in various inflammatory processes .
- Alteration of Intracellular Calcium Levels : The drug affects calcium signaling pathways within cells, potentially influencing various cellular responses, including apoptosis and inflammation .
- Prevention of Thrombus Formation : HCQ may also inhibit platelet aggregation and thrombus formation, contributing to its anti-inflammatory properties .
Research Findings
Numerous studies have investigated the efficacy of HCQ in various contexts, particularly concerning COVID-19. The following table summarizes key findings from significant clinical trials:
Case Studies
Several observational studies have highlighted both the potential benefits and limitations of HCQ:
- Chen et al. (2020) conducted a prospective randomized clinical trial with 62 hospitalized patients, finding that those treated with HCQ had significantly shorter recovery times compared to the control group .
- Sbidian et al. (2020) analyzed data from over 4600 patients and found no difference in mortality rates between those receiving HCQ and those receiving usual care; however, discharge rates were significantly higher in the HCQ group .
Controversies and Limitations
Despite initial hopes for HCQ as a treatment for COVID-19, subsequent large-scale studies have largely discredited its efficacy against the virus:
- The RECOVERY trial , one of the largest studies conducted, concluded that there was no meaningful benefit from HCQ treatment in hospitalized patients .
- Observational studies often suffered from methodological flaws such as small sample sizes and lack of randomization, leading to overinterpretation of results .
Eigenschaften
IUPAC Name |
2-[4-[(7-chloroquinolin-4-yl)amino]pentyl-ethylamino]ethanol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H26ClN3O/c1-3-22(11-12-23)10-4-5-14(2)21-17-8-9-20-18-13-15(19)6-7-16(17)18/h6-9,13-14,23H,3-5,10-12H2,1-2H3,(H,20,21) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XXSMGPRMXLTPCZ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCN(CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl)CCO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H26ClN3O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
747-36-4 (sulfate (1:1) salt) | |
| Record name | Hydroxychloroquine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000118423 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID8023135 | |
| Record name | Hydroxychloroquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023135 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
335.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Hydroxychloroquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015549 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.61e-02 g/L | |
| Record name | Hydroxychloroquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015549 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The exact mechanisms of hydroxychloroquine are unknown. It has been shown that hydroxychloroquine accumulates in the lysosomes of the malaria parasite, raising the pH of the vacuole. This activity interferes with the parasite's ability to proteolyse hemoglobin, preventing the normal growth and replication of the parasite. Hydroxychloroquine can also interfere with the action of parasitic heme polymerase, allowing for the accumulation of the toxic product beta-hematin. Hydroxychloroquine accumulation in human organelles also raise their pH, which inhibits antigen processing, prevents the alpha and beta chains of the major histocompatibility complex (MHC) class II from dimerizing, inhibits antigen presentation of the cell, and reduces the inflammatory response. Elevated pH in the vesicles may alter the recycling of MHC complexes so that only the high affinity complexes are presented on the cell surface. Self peptides bind to MHC complexes with low affinity and so they will be less likely to be presented to autoimmune T cells. Hydroxychloroquine also reduces the release of cytokines like interleukin-1 and tumor necrosis factor, possibly through inhibition of Toll-like receptors. The raised pH in endosomes, prevent virus particles (such as SARS-CoV and SARS-CoV-2) from utilizing their activity for fusion and entry into the cell. Hydroxychloroquine inhibits terminal glycosylation of ACE2, the receptor that SARS-CoV and SARS-CoV-2 target for cell entry. ACE2 that is not in the glycosylated state may less efficiently interact with the SARS-CoV-2 spike protein, further inhibiting viral entry. | |
| Record name | Hydroxychloroquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01611 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
118-42-3 | |
| Record name | Hydroxychloroquine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=118-42-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Hydroxychloroquine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000118423 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Hydroxychloroquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01611 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Hydroxychloroquine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8023135 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Hydroxychloroquine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.003.864 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | HYDROXYCHLOROQUINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4QWG6N8QKH | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Hydroxychloroquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015549 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
89-91, 90 °C | |
| Record name | Hydroxychloroquine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01611 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Hydroxychloroquine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015549 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.
















