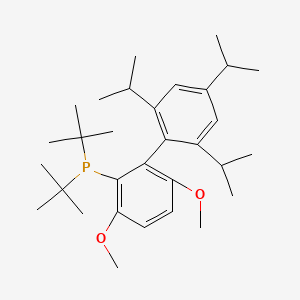
tBuBrettPhos
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Description
TBuBrettPhos, also known as this compound, is a useful research compound. Its molecular formula is C31H49O2P and its molecular weight is 484.705. The purity is usually 95%.
BenchChem offers high-quality this compound suitable for many research applications. Different packaging options are available to accommodate customers' requirements. Please inquire for more information about this compound including the price, delivery time, and more detailed information at [email protected].
Mechanism of Action
Target of Action
tBuBrettPhos is a dialkylbiaryl phosphine ligand . It is primarily targeted towards palladium , a transition metal, and is widely used in palladium-catalyzed cross-coupling reactions .
Mode of Action
This compound interacts with its target, palladium, to form a complex that acts as a precatalyst . This precatalyst promotes cross-coupling reactions more efficiently and exhibits improved reactivity compared to other catalytic systems . The formation of the active catalytic species is efficient, and the ligand to palladium ratio can be accurately controlled .
Biochemical Pathways
The primary biochemical pathway influenced by this compound involves the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds . These bonds are crucial in the synthesis of various organic compounds. The compound’s action can affect multiple downstream effects, including the synthesis of complex organic molecules.
Pharmacokinetics
Its solubility in a wide range of common organic solvents suggests that it could have good distribution properties if used in a biological context.
Result of Action
The result of this compound’s action is the efficient promotion of cross-coupling reactions . This leads to the formation of various organic compounds, including those with C-C, C–N, C–O, C–F, C–CF3, and C–S bonds . It has been used as a precatalyst for the N-arylation of amino acid esters with aryl triflates under mild reaction conditions and minimal racemization of the amino acid ester .
Action Environment
This compound is air-, moisture-, and thermally-stable , suggesting that it can maintain its efficacy and stability under a variety of environmental conditions.
Biological Activity
tBuBrettPhos, a phosphine ligand, has garnered attention in the field of organometallic chemistry due to its significant role in catalysis, particularly in palladium-catalyzed reactions. This article explores the biological activity of this compound, focusing on its applications, mechanisms of action, and relevant research findings.
Chemical Structure and Properties
This compound is characterized by a bulky tert-butyl group that enhances its steric properties, making it a valuable ligand in various catalytic processes. Its structure allows for effective coordination with transition metals, facilitating reactions such as cross-coupling and C–S bond formation.
Biological Activity Overview
While this compound is primarily recognized for its catalytic applications, recent studies have begun to explore its biological implications. The biological activity can be categorized into several key areas:
- Catalytic Efficiency : this compound has shown high yields in palladium-catalyzed reactions, which are crucial in synthesizing biologically active compounds.
- Inhibition Studies : Research indicates potential inhibitory effects on certain biological pathways, although specific studies on direct biological targets remain limited.
1. Catalytic Applications
This compound has been extensively studied for its role in catalyzing C–S cross-coupling reactions. A study demonstrated that using this compound as a ligand resulted in yields exceeding 97% when applied to aryl thiol arylation reactions (Table 1) .
| Reaction Type | Yield (%) | Conditions |
|---|---|---|
| Aryl Thiol Arylation | 97% | Room temperature, 2 hours |
| C–N Cross-Coupling | 91% | Varying aryl halides |
2. Inhibition of CYP Enzymes
A study evaluated the inhibition potential of various phosphine ligands, including this compound, on cytochrome P450 enzymes (CYPs). While specific IC50 values for this compound were not reported, the study highlighted its role in modulating enzyme activity indirectly through catalytic pathways .
3. Mechanistic Insights
Research into the mechanistic aspects of this compound revealed that it enhances the stability of palladium catalysts under various reaction conditions. This stability is crucial for maintaining enzyme functionality during catalytic processes .
Discussion
The biological activity of this compound is primarily linked to its efficacy as a ligand in catalysis rather than direct biological interactions. Its ability to facilitate high-yield reactions makes it an essential compound in synthesizing pharmaceuticals and other biologically relevant molecules. However, more focused research is needed to elucidate any direct biological effects or interactions with specific biological targets.
Properties
IUPAC Name |
ditert-butyl-[3,6-dimethoxy-2-[2,4,6-tri(propan-2-yl)phenyl]phenyl]phosphane |
Source


|
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C31H49O2P/c1-19(2)22-17-23(20(3)4)27(24(18-22)21(5)6)28-25(32-13)15-16-26(33-14)29(28)34(30(7,8)9)31(10,11)12/h15-21H,1-14H3 |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
REWLCYPYZCHYSS-UHFFFAOYSA-N |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C1=CC(=C(C(=C1)C(C)C)C2=C(C=CC(=C2P(C(C)(C)C)C(C)(C)C)OC)OC)C(C)C |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C31H49O2P |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID70657989 |
Source


|
| Record name | Di-tert-butyl[3,6-dimethoxy-2',4',6'-tri(propan-2-yl)[1,1'-biphenyl]-2-yl]phosphane | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID70657989 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
484.7 g/mol |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1160861-53-9 |
Source


|
| Record name | Di-tert-butyl[3,6-dimethoxy-2',4',6'-tri(propan-2-yl)[1,1'-biphenyl]-2-yl]phosphane | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID70657989 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Di-tert-butyl(2',4',6'-triisopropyl-3,6-dimethoxy-[1,1'-biphenyl]-2-yl)phosphine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













