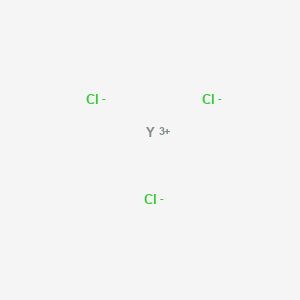
Yttrium chloride
- Click on QUICK INQUIRY to receive a quote from our team of experts.
- With the quality product at a COMPETITIVE price, you can focus more on your research.
Overview
Description
Yttrium chloride is an inorganic compound composed of yttrium and chloride. It exists in two forms: the hydrate (YCl₃·6H₂O) and the anhydrous form (YCl₃). Both forms are colorless salts that are highly soluble in water and deliquescent . This compound is used in various applications, including as a precursor for the synthesis of other yttrium compounds and in materials science.
Mechanism of Action
Target of Action
Yttrium(III) chloride, also known as Yttrium chloride(III), is an inorganic compound of yttrium and chloride . It is used as a precursor to synthesize yttrium-based nanomaterials such as yttrium aluminum garnet nanopowder and organometallic yttrium complexes . These materials find application in the field of catalysis, electroluminescent devices, and superconductors . Therefore, the primary targets of Yttrium(III) chloride are these yttrium-based nanomaterials and their associated applications.
Mode of Action
Yttrium(III) chloride reacts with potassium alkoxotitanates to form precursors for titanium-containing ceramics . It is also used in the production of yttrium alkyl alkoxide complexes . The compound interacts with its targets through chemical reactions, leading to the formation of new compounds with desired properties.
Biochemical Pathways
It is known to be involved in the synthesis of yttrium-based nanomaterials and organometallic yttrium complexes , which can have various downstream effects depending on their specific applications.
Pharmacokinetics
It has been documented that most of the yttrium administered was distributed into liver, bone, and spleen . Yttrium disappeared from the blood within 1 day but was retained in the organs for a long time . These findings suggest that Yttrium(III) chloride may have low bioavailability due to its rapid clearance from the blood and long retention in the organs.
Result of Action
The molecular and cellular effects of Yttrium(III) chloride’s action largely depend on its application. For instance, when used as a precursor to synthesize yttrium-based nanomaterials, the result of its action is the formation of these nanomaterials with desired properties .
Action Environment
The action, efficacy, and stability of Yttrium(III) chloride can be influenced by various environmental factors. For example, its solubility in water and other solvents can affect its availability for reactions . Moreover, its reactivity with other substances can be influenced by factors such as temperature and pH.
Biochemical Analysis
Biochemical Properties
The biological effects of Yttrium refer to the activity, behavior, and toxicity of Yttrium element or compounds in cells, tissues, organs, and organisms . Yttrium is present in low abundance in soil, water bodies, and organisms . It is not an essential element for organisms and its bioavailability is very low .
Cellular Effects
Yttrium(III) chloride has been found to have acute hepatic injury and transient increase of plasma Ca following the injection . A significant and tremendous amount of Ca was deposited in the liver (over 10-fold) and spleen (over 100-fold), while Ca concentration was only slightly increased in the lung and kidney (less than 1.5-fold) . These results indicate that liver and spleen are primary target organs of intravenous-injected Yttrium(III) chloride .
Molecular Mechanism
Yttrium(III) chloride is taken up by phagocytic cells in the liver and spleen . Electron microscopic analyzes revealed that the colloidal yttrium-containing material was taken up by phagocytic cells in the liver and spleen .
Temporal Effects in Laboratory Settings
The elimination half-time of liver Y was 144 days at a dose of 1 mg Y/rat . Yttrium disappeared from the blood within 1 day but was retained in the organs for a long time .
Dosage Effects in Animal Models
The acute toxicity of Yttrium ion (Y3+), the deposition, retention, metabolism, and clearance have been documented in the previous reports . Hirano et al. studied time-course and dose-related changes in tissue distribution, subcellular localization, clearance, and acute toxicity of intravenous-injected Yttrium(III) chloride in rats .
Metabolic Pathways
Changes in Ca concentrations in liver, spleen, and lungs were in accordance with those of Yttrium(III) chloride . Two mechanisms of REE metabolism in the case of intravenous administration are suggested: (1) The REEs may be transported partly contained in serum protein and partly incorporated into phagocytes, and taken mostly into the reticuloendothelial system by the phagocytic mechanism and deposited there. (2) The major route of excretion of REEs may be biliary excretion and the REEs might be excreted gradually into feces .
Transport and Distribution
Yttrium(III) chloride is transported and distributed within cells and tissues . It is taken up by phagocytic cells in the liver and spleen .
Subcellular Localization
The subcellular localization of Yttrium(III) chloride is within the phagocytic cells in the liver and spleen . Electron microscopic analyzes revealed that the colloidal yttrium-containing material was taken up by phagocytic cells in the liver and spleen .
Preparation Methods
Yttrium chloride is often prepared by the “ammonium chloride route,” starting from either yttrium oxide (Y₂O₃) or hydrated chloride or oxychloride . The synthetic route involves the following reactions:
From Y₂O₃: [ 10 \text{NH}_4\text{Cl} + \text{Y}_2\text{O}_3 \rightarrow 2 (\text{NH}_4)_2[\text{YCl}_5] + 6 \text{NH}_3 + 3 \text{H}_2\text{O} ]
From YCl₃·6H₂O: [ \text{YCl}_3·6\text{H}_2\text{O} + 2 \text{NH}_4\text{Cl} \rightarrow (\text{NH}_4)_2[\text{YCl}_5] + 6 \text{H}_2\text{O} ]
The pentachloride decomposes thermally to yield this compound(III): [ (\text{NH}_4)_2[\text{YCl}_5] \rightarrow 2 \text{NH}_4\text{Cl} + \text{YCl}_3 ]
Chemical Reactions Analysis
Yttrium chloride undergoes various types of chemical reactions, including:
Oxidation: this compound can be oxidized to form yttrium oxychloride (YClO).
Reduction: It can be reduced to elemental yttrium using strong reducing agents.
Substitution: This compound reacts with potassium alkoxotitanates to form precursors for titanium-containing ceramics.
Common reagents and conditions used in these reactions include ammonium chloride, potassium alkoxotitanates, and high temperatures. Major products formed from these reactions include yttrium oxychloride and yttrium alkyl alkoxide complexes.
Scientific Research Applications
Materials Science
Yttrium Chloride in Ceramics and Superconductors
this compound is crucial in the synthesis of yttrium-based ceramics and superconductors. It serves as a precursor for materials like Yttrium Barium Copper Oxide (YBCO), which is renowned for its high-temperature superconducting properties.
| Application | Description |
|---|---|
| Ceramics | Used to create stable and durable ceramic materials, essential for high-performance applications. |
| Superconductors | Facilitates the production of superconducting materials that operate at higher temperatures compared to traditional superconductors. |
Phosphor Production
This compound is integral to the manufacturing of phosphors used in light-emitting diode (LED) technologies and display systems. Its ability to enhance brightness and energy efficiency makes it a preferred choice in the electronics industry.
| Application | Description |
|---|---|
| LEDs | Enhances brightness and energy efficiency in LED lights. |
| Display Technologies | Key ingredient in phosphors for televisions and monitors, improving color quality. |
Catalysis
In organic synthesis, this compound acts as a catalyst to improve reaction rates and selectivity. Its application in catalysis is vital for developing more efficient chemical processes.
| Application | Description |
|---|---|
| Catalysis | Used to facilitate various chemical reactions, enhancing efficiency and selectivity in organic synthesis. |
Biomedical Applications
This compound has emerging applications in biomedicine, particularly in targeted drug delivery systems and as a contrast agent in medical imaging. Its unique properties allow for clearer imaging results, which are crucial for diagnostics.
| Application | Description |
|---|---|
| Drug Delivery | Potential use in targeted therapies to improve drug efficacy while minimizing side effects. |
| Medical Imaging | Serves as a contrast agent in imaging techniques like MRI, enhancing image clarity. |
Nuclear Medicine
Yttrium-90 chloride, a radioisotope derived from this compound, plays a critical role in radioimmunotherapy for cancer treatment. It allows for targeted radiation therapy that minimizes damage to surrounding healthy tissues.
| Application | Description |
|---|---|
| Radioimmunotherapy | Utilized to deliver targeted radiation to cancer cells while sparing healthy tissues. |
| Radioembolization | Used in treating liver cancer by delivering radiation directly to tumors through blood vessels. |
Case Study 1: this compound in Superconductors
Research has demonstrated that YBCO synthesized using this compound exhibits superior performance at elevated temperatures compared to traditional superconductors. This advancement has implications for power grids and magnetic resonance imaging technologies .
Case Study 2: Cytotoxicity Studies
A study investigating the cytotoxic effects of this compound on cardiomyocytes revealed that exposure can lead to DNA damage via reactive oxygen species overproduction . This finding underscores the importance of understanding the biological impacts of yttrium compounds as they are increasingly used in medical applications.
Comparison with Similar Compounds
Yttrium chloride can be compared with other similar compounds, such as:
- Yttrium fluoride(III) (YF₃)
- Yttrium bromide(III) (YBr₃)
- Yttrium iodide(III) (YI₃)
- Scandium chloride(III) (ScCl₃)
- Lutetium chloride(III) (LuCl₃)
This compound is unique due to its high solubility in water and its ability to form various yttrium-based materials. Its layered crystal structure, shared by compounds like aluminum chloride (AlCl₃), also contributes to its distinct properties .
Biological Activity
Yttrium chloride (YCl₃) is a compound of yttrium, a rare earth element, that has garnered attention for its biological activity, particularly its effects on cellular processes and potential applications in medical treatments. This article synthesizes findings from various studies to provide a comprehensive overview of the biological activity of this compound, including its mechanisms of action, cytotoxicity, and implications for health.
Recent research has highlighted the cytotoxic effects of YCl₃ on various cell types. A significant study demonstrated that long-term exposure to YCl₃ induces apoptosis in Leydig cells, which are crucial for testosterone production in the testes. The mechanism involves the upregulation of the Ca²⁺/IP₃R₁/CaMKII signaling pathway, leading to increased intracellular calcium levels and activation of apoptotic pathways .
Key Findings:
- Cell Apoptosis : YCl₃ treatment resulted in increased expression of cleaved caspase-3 and decreased Bcl-2 levels, indicating a shift towards apoptosis in Leydig cells .
- Calcium Signaling : The compound enhances cytosolic Ca²⁺ concentrations, which is crucial for activating apoptotic signals .
Cytotoxicity Studies
This compound's cytotoxicity has been evaluated in various models, revealing significant effects on cell viability:
| Study | Cell Type | IC₅₀ (µM) | Effects Observed |
|---|---|---|---|
| Leydig Cells | 300 | Induction of apoptosis | |
| MDA-MB-231 (Breast Cancer) | 74.4 | Selective cytotoxicity through ROS generation |
In a study focusing on breast cancer cells (MDA-MB-231), YCl₃ exhibited selective cytotoxic effects, primarily through the generation of reactive oxygen species (ROS), leading to DNA damage and apoptosis .
Case Studies
- Reproductive Toxicity : In an animal model, chronic exposure to YCl₃ resulted in significant testicular damage characterized by cellular apoptosis and alterations in hormone production. This was associated with upregulation of apoptotic markers and disruption of normal Leydig cell function .
- Hepatic Effects : Another study noted that intravenous administration of YCl₃ led to accumulation in the liver and spleen, causing transient increases in liver enzymes (AST and ALT), indicative of hepatic stress . The compound was found to form colloidal materials within phagocytic cells, impacting calcium metabolism significantly.
Implications for Medical Applications
This compound has been explored for its potential therapeutic applications, particularly in targeted therapies such as radioisotope treatment for cancer. The ability to accumulate selectively in certain tissues suggests that it could be utilized for localized treatment strategies while minimizing systemic toxicity.
Potential Applications:
- Cancer Treatment : Leveraging its cytotoxic properties against cancer cells while minimizing damage to normal tissues.
- Radiopharmaceuticals : As a carrier for radioactive isotopes, enhancing the efficacy of radiotherapy through targeted delivery.
Properties
CAS No. |
10361-92-9 |
|---|---|
Molecular Formula |
Cl3Y |
Molecular Weight |
195.26 g/mol |
IUPAC Name |
trichloroyttrium |
InChI |
InChI=1S/3ClH.Y/h3*1H;/q;;;+3/p-3 |
InChI Key |
PCMOZDDGXKIOLL-UHFFFAOYSA-K |
SMILES |
[Cl-].[Cl-].[Cl-].[Y+3] |
Canonical SMILES |
Cl[Y](Cl)Cl |
Key on ui other cas no. |
10361-92-9 |
physical_description |
WetSolid |
Related CAS |
10025-94-2 (hexahydrate) |
Synonyms |
YCl3 yttrium chloride yttrium chloride hexahydrate yttrium chloride, (88)Y-labeled yttrium chloride, (90)Y-labeled yttrium chloride, (91)Y-labeled |
Origin of Product |
United States |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















