Albendazole-D3
Descripción general
Descripción
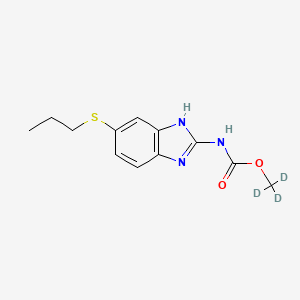
Albendazole-D3 is a deuterated form of Albendazole, a benzimidazole derivative widely used as an anthelmintic agent. This compound is chemically known as trideuteriomethyl N-(5-propylsulfanyl-1H-benzimidazol-2-yl)carbamate. The deuterium atoms replace the hydrogen atoms in the methyl group, which can enhance the compound’s stability and metabolic profile.
Métodos De Preparación
Synthetic Routes and Reaction Conditions
The synthesis of Albendazole-D3 involves the incorporation of deuterium atoms into the Albendazole molecule. One common method is the deuterium exchange reaction, where Albendazole is treated with deuterated reagents under specific conditions to replace hydrogen atoms with deuterium. This process typically involves the use of deuterated solvents and catalysts to facilitate the exchange.
Industrial Production Methods
Industrial production of this compound follows similar principles but on a larger scale. The process involves the use of high-purity deuterated reagents and advanced catalytic systems to ensure efficient and consistent deuterium incorporation. The final product is purified using techniques such as crystallization and chromatography to achieve the desired purity and isotopic enrichment.
Análisis De Reacciones Químicas
Types of Reactions
Albendazole-D3 undergoes various chemical reactions, including:
Oxidation: The compound can be oxidized to form sulfoxides and sulfones.
Reduction: Reduction reactions can convert this compound to its corresponding amine derivatives.
Substitution: Nucleophilic substitution reactions can introduce different functional groups into the molecule.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include hydrogen peroxide and m-chloroperbenzoic acid.
Reduction: Reducing agents such as lithium aluminum hydride and sodium borohydride are used.
Substitution: Reagents like alkyl halides and acyl chlorides are employed under basic or acidic conditions.
Major Products Formed
Oxidation: Sulfoxides and sulfones.
Reduction: Amine derivatives.
Substitution: Various substituted benzimidazole derivatives.
Aplicaciones Científicas De Investigación
Pharmacokinetics and Metabolism
One of the key applications of Albendazole-D3 is in pharmacokinetic studies. A recent study characterized the excretion patterns of albendazole metabolites in human saliva, utilizing this compound as an internal standard for high-performance liquid chromatography (HPLC) analysis. This method demonstrated that saliva could be a viable biological matrix for assessing compliance in mass drug administration (MDA) campaigns against soil-transmitted helminths (STHs) .
Key Findings:
- Detection Limits: The limit of detection for albendazole sulfoxide (ABZSO) was established at 0.01 µg/mL in saliva.
- Pharmacokinetic Parameters: The peak concentration was observed at 4 hours post-treatment, with a significant correlation between saliva and dried blood spot (DBS) samples (Pearson r-value: 0.907) .
Therapeutic Efficacy
Albendazole has been extensively studied for its efficacy against various parasitic infections. In a case study involving orbital cysticercosis, a three-day regimen of albendazole was shown to be curative without recurrence . The study documented ultrasonography findings that illustrated the drug's effectiveness in reducing cyst size and alleviating symptoms.
Case Study Summary:
- Participants: 10 cases of orbital cysticercosis.
- Treatment Protocol: Oral prednisolone and albendazole administered over three days.
- Outcome: Complete resolution of symptoms and cysts observed in all patients by day 30 .
Combination Therapies
Research has also explored the combination of albendazole with other antiparasitic agents. A study on the pharmacokinetics of moxidectin combined with albendazole indicated that this triple-drug therapy could enhance treatment efficacy without increasing adverse events . This approach is particularly relevant for areas with high transmission rates of filarial diseases.
Analytical Methods Development
The development of sensitive analytical methods for detecting albendazole and its metabolites continues to be a focus area. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been optimized for simultaneous determination of albendazole and its active metabolite in human plasma, using this compound as an internal standard .
Methodological Insights:
- Sample Preparation: Solid-phase extraction was utilized for efficient analyte isolation.
- Performance Metrics: The method demonstrated high sensitivity and specificity, crucial for clinical pharmacokinetic studies.
Environmental Impact Studies
Albendazole's environmental fate is also an area of concern due to its potential impact on non-target organisms. Studies investigating advanced oxidation processes (AOPs) for the degradation of albendazole have highlighted its persistence in aquatic environments, emphasizing the need for effective wastewater treatment solutions .
Mecanismo De Acción
Albendazole-D3 exerts its effects by binding to the colchicine-sensitive site of tubulin, inhibiting its polymerization into microtubules. This disruption of microtubule formation leads to the degeneration of the intestinal cells of the parasites, ultimately causing their death. The deuterium atoms in this compound enhance the compound’s stability and metabolic profile, potentially leading to improved therapeutic outcomes.
Comparación Con Compuestos Similares
Similar Compounds
Mebendazole: Another benzimidazole derivative with similar anthelmintic properties.
Thiabendazole: A benzimidazole compound used to treat parasitic infections.
Fenbendazole: A broad-spectrum anthelmintic used in veterinary medicine.
Uniqueness
Albendazole-D3 is unique due to the presence of deuterium atoms, which enhance its stability and metabolic profile. This makes it a valuable compound for research and therapeutic applications, offering potential advantages over non-deuterated analogs in terms of efficacy and safety.
Actividad Biológica
Albendazole-D3 (ABZ-D3) is a deuterated form of the widely used anthelmintic drug albendazole (ABZ). It is primarily utilized in pharmacokinetic studies to trace the metabolism and distribution of albendazole and its metabolites in biological systems. This article reviews the biological activity of ABZ-D3, focusing on its pharmacological effects, metabolic pathways, and clinical implications.
1. Pharmacological Profile
Albendazole is known for its efficacy against a variety of parasitic infections, particularly those caused by soil-transmitted helminths (STH) such as Ascaris lumbricoides and Trichuris trichiura. The biological activity of ABZ-D3 can be understood through its pharmacokinetics and metabolism:
- Metabolism : After oral administration, ABZ is rapidly metabolized in the liver to its active metabolite, albendazole sulfoxide (ABZSO), which is responsible for the drug's anthelmintic action. ABZSO undergoes further conversion to albendazole sulfone (ABZSO2), which lacks significant anthelmintic activity .
- Pharmacokinetics : Studies indicate that ABZSO is approximately 70% bound to plasma proteins and is widely distributed throughout the body, detectable in urine, cerebrospinal fluid, liver, bile, cyst walls, and cyst fluids . The pharmacokinetic properties of ABZ have been characterized in various populations, including healthy subjects and patients with parasitic infections.
2.1 Anthelmintic Activity
ABZ-D3 has been evaluated for its efficacy against STH infections. A study involving a triple-dose regimen of albendazole demonstrated significant cure rates for Ascaris lumbricoides and hookworm infections, with egg reduction rates exceeding 90% . The study highlighted that children under 18 years old were particularly susceptible to STH infections due to poor sanitation practices.
2.2 Cytotoxicity Studies
Research comparing the cytotoxic effects of ABZ on various cell lines revealed that Balb/c 3T3 cells were most sensitive to ABZ, followed by FaO and HepG2 cells. The effective concentration (EC50) values indicated that Balb/c 3T3 cells had an EC50 of 0.2 µg/mL, while FaO and HepG2 cells had higher EC50 values of 1.0 µg/mL and 6.4 µg/mL, respectively . This suggests that non-metabolizing cells exhibit higher sensitivity to the parent compound compared to metabolizing hepatoma cells.
3. Metabolic Pathways
Albendazole undergoes extensive hepatic metabolism. The primary metabolic pathways include:
- Conversion to Albendazole Sulfoxide : This metabolite exhibits significant anthelmintic activity and is responsible for much of the therapeutic effect observed with albendazole treatment.
- Further Metabolism to Albendazole Sulfone : This metabolite has minimal anthelmintic activity but may contribute to the overall pharmacological profile .
The following table summarizes key metabolites and their respective activities:
| Metabolite | Activity Level | Binding Affinity |
|---|---|---|
| Albendazole | High | Moderate |
| Albendazole Sulfoxide | High | 70% bound to proteins |
| Albendazole Sulfone | Low | N/A |
4. Clinical Implications
The use of ABZ-D3 as a tracer in clinical studies has facilitated a deeper understanding of albendazole's pharmacokinetics. For instance, research has shown that ABZSO levels can be monitored in saliva as a biomarker for compliance in mass drug administration (MDA) programs targeting STH infections . This approach allows for more accurate assessments of drug efficacy and patient adherence.
5. Case Studies
Several case studies have demonstrated the effectiveness of albendazole in treating parasitic infections:
- A longitudinal study conducted among indigenous communities in Malaysia reported high prevalence rates of STH infections and evaluated the effectiveness of a triple-dose regimen of albendazole . The results indicated a significant reduction in infection rates post-treatment.
- Another clinical trial assessed the use of albendazole in combination with other antiparasitic agents, revealing enhanced efficacy compared to monotherapy .
Propiedades
IUPAC Name |
trideuteriomethyl N-(6-propylsulfanyl-1H-benzimidazol-2-yl)carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C12H15N3O2S/c1-3-6-18-8-4-5-9-10(7-8)14-11(13-9)15-12(16)17-2/h4-5,7H,3,6H2,1-2H3,(H2,13,14,15,16)/i2D3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HXHWSAZORRCQMX-BMSJAHLVSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCSC1=CC2=C(C=C1)N=C(N2)NC(=O)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])OC(=O)NC1=NC2=C(N1)C=C(C=C2)SCCC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C12H15N3O2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID401016410 | |
| Record name | Albendazole-(methyl-d3) | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID401016410 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
268.35 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1353867-92-1 | |
| Record name | ||
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1353867-92-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Albendazole-(methyl-d3) | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID401016410 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1353867-92-1 | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
Synthesis routes and methods I
Procedure details













Synthesis routes and methods II
Procedure details








Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.













