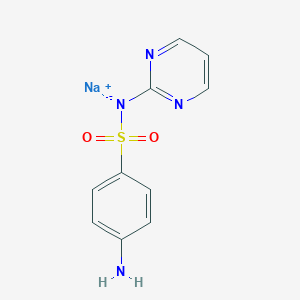
Sulfadiazina sódica
Descripción general
Descripción
Sulfadiazine sodium is a sulfonamide antibiotic used to treat a variety of bacterial infections. It is particularly effective against urinary tract infections, trachoma, and chancroid . Sulfadiazine sodium is also used in combination with pyrimethamine to treat toxoplasmosis in patients with acquired immunodeficiency syndrome and in newborns with congenital infections .
Aplicaciones Científicas De Investigación
Introduction to Sulfadiazine Sodium
Sulfadiazine sodium is a sulfonamide antibiotic that plays a significant role in both human and veterinary medicine. It was developed in the 1940s as a more effective and less toxic alternative to sulfapyridine. The compound acts as a bacteriostatic agent by inhibiting the enzyme dihydropteroate synthetase, which is essential for folic acid synthesis in bacteria. This mechanism of action allows sulfadiazine sodium to exhibit broad-spectrum antibacterial activity against various gram-positive and gram-negative bacteria, making it a valuable tool in clinical microbiology and treatment protocols.
Clinical Applications
Antimicrobial Susceptibility Testing
Sulfadiazine sodium is commonly utilized in microbiological laboratories for antimicrobial susceptibility testing. It is part of various testing panels and methods, such as disc diffusion and minimum inhibitory concentration (MIC) assays, to determine the effectiveness of antibiotics against specific pathogens. This application is crucial for guiding appropriate antibiotic therapy in infected patients .
Treatment of Toxoplasmosis
In clinical settings, sulfadiazine sodium is often used in combination with pyrimethamine to treat toxoplasmosis, particularly in immunocompromised patients such as those with HIV/AIDS. This combination therapy has shown efficacy in reducing the severity of the infection and preventing complications associated with toxoplasmosis .
Intravenous Administration
Sulfadiazine sodium can also be administered intravenously when rapid therapeutic levels are required, such as in cases of severe pneumococcal pneumonia. This method allows for immediate drug action and higher bioavailability compared to oral administration .
Veterinary Medicine
Livestock Treatment
In veterinary applications, sulfadiazine sodium is used to treat bacterial infections in livestock. Its effectiveness against common pathogens makes it a staple in veterinary practices for managing respiratory and gastrointestinal infections .
Antimicrobial Resistance Studies
Research has been conducted on the use of sulfadiazine sodium in livestock farming, focusing on antimicrobial resistance (AMR). Studies indicate that improper use of antibiotics like sulfadiazine can contribute to AMR, necessitating careful management practices among farmers .
Agricultural Applications
Herbicide Potential
Sulfadiazine sodium has been explored for its herbicidal properties, specifically its ability to inhibit folic acid synthesis in plants. This characteristic allows it to serve as a selective marker in the genetic transformation processes of crops like tobacco and algae, enhancing the efficiency of creating transgenic plants .
Case Study: Toxoplasmosis Treatment
A study investigated the metabolomic changes associated with Toxoplasma gondii infection in mice treated with sulfadiazine sodium. The findings revealed significant alterations in host metabolism, highlighting both therapeutic effects and potential side effects such as nausea and rash . This underscores the importance of monitoring metabolic responses during treatment.
Research on Antimicrobial Use in Livestock
A comprehensive study analyzed the drivers behind antibiotic use in small-scale poultry farming, revealing that sulfadiazine sodium is frequently used despite regulations against its use in feed. The findings emphasized the need for better governance and education on responsible antibiotic use to combat AMR effectively .
Summary Table of Applications
| Application Area | Details |
|---|---|
| Clinical Microbiology | Used for antimicrobial susceptibility testing; treatment of infections like toxoplasmosis |
| Veterinary Medicine | Treats bacterial infections in livestock; studies on AMR related to its use |
| Agricultural Research | Explored as a herbicide; used as a marker gene for transgenic plant development |
Mecanismo De Acción
Target of Action
Sulfadiazine sodium primarily targets the bacterial enzyme dihydropteroate synthetase . This enzyme plays a crucial role in the synthesis of folic acid, which is essential for bacterial growth and survival .
Mode of Action
Sulfadiazine sodium acts as a competitive inhibitor of dihydropteroate synthetase . By binding to this enzyme, it prevents the proper processing of para-aminobenzoic acid (PABA), a precursor required for folic acid synthesis . This inhibition disrupts the synthesis of folic acid, thereby inhibiting bacterial growth .
Biochemical Pathways
The primary biochemical pathway affected by sulfadiazine sodium is the folic acid synthesis pathway in bacteria . By inhibiting dihydropteroate synthetase, sulfadiazine sodium disrupts this pathway, leading to a deficiency in folic acid. Since folic acid is necessary for the synthesis of nucleic acids and proteins, its deficiency impairs bacterial growth and proliferation .
Pharmacokinetics
They are known to bind to plasma proteins and are metabolized in the liver . The metabolites and unchanged drug are primarily excreted in the urine .
Result of Action
The molecular effect of sulfadiazine sodium’s action is the inhibition of folic acid synthesis, which leads to a deficiency in folic acid within the bacterial cell . On a cellular level, this results in impaired synthesis of nucleic acids and proteins, leading to inhibited bacterial growth and proliferation .
Action Environment
Environmental factors can influence the action of sulfadiazine sodium. For instance, the presence of organic matter, such as humic acid, in the soil can affect the sorption and transport of sulfadiazine, thereby influencing its environmental fate and eco-toxicity . Furthermore, the pH and ionic strength of the solution can affect the sorption of sulfadiazine on humic acid . These factors can potentially influence the efficacy and stability of sulfadiazine sodium in the environment .
Análisis Bioquímico
Biochemical Properties
Sulfadiazine sodium plays a significant role in biochemical reactions. It interacts with various enzymes, proteins, and other biomolecules. For instance, it forms inclusion compounds with β-cyclodextrin and its derivatives . These interactions have been confirmed through isothermal titration calorimetry and nuclear magnetic resonance experiments .
Cellular Effects
The effects of Sulfadiazine sodium on various types of cells and cellular processes are profound. It influences cell function, including impacts on cell signaling pathways, gene expression, and cellular metabolism. For instance, the inclusion compounds of Sulfadiazine sodium with β-cyclodextrin and its derivatives have shown increased efficiency against Staphylococcus aureus and Pseudomonas aeruginosa compared to the free Sulfadiazine sodium molecule .
Métodos De Preparación
Synthetic Routes and Reaction Conditions
Sulfadiazine sodium is synthesized by the diazotization of the primary amine group of sulfadiazine with sodium nitrite and hydrochloric acid, followed by coupling with γ-resorsolic acid in an alkaline medium of sodium hydroxide . The compound is then purified and crystallized to obtain the final product.
Industrial Production Methods
In industrial settings, sulfadiazine sodium is produced by adding sulfadiazine, trimethoprim, and enrofloxacin to a mixture of polyethylene glycol and propylene glycol, followed by heating and stirring . The solution is then standardized, and the pH is adjusted to 10.5-11 to obtain the final product .
Análisis De Reacciones Químicas
Types of Reactions
Sulfadiazine sodium undergoes various chemical reactions, including:
Reduction: The compound can be reduced under specific conditions, although this is less common.
Substitution: Sulfadiazine sodium can undergo substitution reactions, particularly in the presence of strong nucleophiles.
Common Reagents and Conditions
Oxidation: Common reagents include potassium monopersulfate (Oxone) and UV light.
Substitution: Strong nucleophiles such as sodium hydroxide are often used.
Major Products
The major products formed from these reactions include various oxidized and substituted derivatives of sulfadiazine sodium, which can have different pharmacological properties .
Comparación Con Compuestos Similares
Sulfadiazine sodium is part of the sulfonamide class of antibiotics, which includes other compounds such as sulfamethoxazole and sulfisoxazole . Compared to these similar compounds, sulfadiazine sodium has a broader spectrum of activity and is more effective against certain types of bacteria . Additionally, it is often used in combination with other drugs, such as pyrimethamine, to enhance its effectiveness .
List of Similar Compounds
- Sulfamethoxazole
- Sulfisoxazole
- Sulfapyridine
- Sulfacetamide
Actividad Biológica
Sulfadiazine sodium, a sulfonamide antibiotic, has garnered significant attention due to its diverse biological activities and applications in medical treatments. This article provides a comprehensive overview of its biological activity, including its mechanisms of action, therapeutic uses, and recent research findings.
Sulfadiazine sodium is characterized by its sulfonamide group, which plays a crucial role in its antibacterial activity. The compound inhibits bacterial growth by interfering with the synthesis of folic acid, an essential nutrient for bacterial DNA synthesis. This mechanism is achieved through competitive inhibition of the enzyme dihydropteroate synthase, which catalyzes the condensation of para-aminobenzoic acid (PABA) with pteridine to form dihydropteroic acid, a precursor in folate synthesis.
Therapeutic Applications
Sulfadiazine sodium is primarily used for treating various infections and conditions, including:
- Urinary Tract Infections (UTIs) : It effectively combats bacterial infections in the urinary system.
- Toxoplasmosis : It is often used in combination with pyrimethamine to treat this parasitic infection.
- Malaria : Sulfadiazine has been utilized as part of combination therapies for malaria treatment.
- Burn Wound Management : Silver sulfadiazine cream is widely used for preventing infections in burn wounds.
Recent Research Findings
Recent studies have explored the efficacy and potential of sulfadiazine sodium and its derivatives. Below are key findings from various research articles:
- Antiproliferative Activity : A study synthesized new sulfadiazine derivatives that exhibited potent antiproliferative effects against cancer cell lines (A431, A549, H1975). The most effective compounds demonstrated IC50 values ranging from 2.31 to 7.56 μM, significantly lower than those of known EGFR inhibitors like erlotinib and gefitinib .
- Urinary Excretion Studies : Research on the urinary excretion patterns of sulfadiazine indicated that factors such as urine pH significantly influence drug output. Increased urine pH led to enhanced excretion rates, highlighting the importance of hydration and urine alkalinity in optimizing drug elimination .
- Comparative Efficacy in Wound Care : A clinical trial compared silver sulfadiazine with povidone-iodine and saline in treating pressure ulcers. The results showed that silver sulfadiazine cream reduced bacterial counts to acceptable levels more effectively than the other treatments, indicating its superior antimicrobial properties .
Table 1: Antiproliferative Activity of Sulfadiazine Derivatives
| Compound | Cell Line | IC50 Value (μM) |
|---|---|---|
| 8 | A431 | 2.31 |
| 12 | A549 | 5.67 |
| 14 | H1975 | 7.56 |
Table 2: Effects of Urinary pH on Sulfadiazine Excretion
| Urinary pH | Average Sulfadiazine Excretion (mg/hr) |
|---|---|
| <6 | 200 |
| 7 | 340 |
| >8 | 516 |
Case Studies
- Case Study on Toxoplasmosis Treatment : In a clinical setting, sulfadiazine was administered alongside pyrimethamine to patients diagnosed with toxoplasmosis. The combination therapy resulted in significant improvements in patient symptoms and reductions in parasitic load.
- Burn Wound Management : A cohort study involving patients with severe burns treated with silver sulfadiazine showed a marked decrease in infection rates and improved healing times compared to traditional methods.
Propiedades
Número CAS |
547-32-0 |
|---|---|
Fórmula molecular |
C10H10N4NaO2S |
Peso molecular |
273.27 g/mol |
Nombre IUPAC |
sodium;(4-aminophenyl)sulfonyl-pyrimidin-2-ylazanide |
InChI |
InChI=1S/C10H10N4O2S.Na/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10;/h1-7H,11H2,(H,12,13,14); |
Clave InChI |
RKISJHYCSQKDTK-UHFFFAOYSA-N |
SMILES |
C1=CN=C(N=C1)[N-]S(=O)(=O)C2=CC=C(C=C2)N.[Na+] |
SMILES canónico |
C1=CN=C(N=C1)NS(=O)(=O)C2=CC=C(C=C2)N.[Na] |
Key on ui other cas no. |
547-32-0 |
Pictogramas |
Irritant; Health Hazard |
Sinónimos |
ulfadiazin-natrium |
Origen del producto |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















