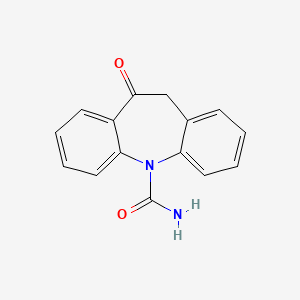
Oxcarbazepina
Descripción general
Descripción
La oxcarbazepina es un medicamento que se utiliza principalmente para tratar la epilepsia. Es un derivado estructural de la carbamazepina y se conoce por sus propiedades anticonvulsivas. La this compound se comercializa bajo varios nombres comerciales, incluidos Trileptal y Oxtellar XR. Se utiliza para controlar las convulsiones de inicio parcial tanto en adultos como en niños .
Mecanismo De Acción
La oxcarbazepina y su metabolito activo, MHD, ejercen sus efectos bloqueando los canales de sodio sensibles al voltaje. Esta acción estabiliza las membranas neuronales hiperexcitadas, inhibe el disparo neuronal repetitivo y reduce la propagación de los impulsos sinápticos. Estos mecanismos son cruciales para prevenir la propagación de las convulsiones .
Compuestos similares:
Carbamazepina: El compuesto padre del que se deriva la this compound.
Eslicarbazepina: Otro derivado con propiedades anticonvulsivas similares.
Lamotrigina: Un anticonvulsivo con una estructura química diferente pero con usos terapéuticos similares .
Comparación:
This compound vs. Carbamazepina: La this compound tiene un mejor perfil de efectos secundarios y menos interacciones medicamentosas en comparación con la carbamazepina.
This compound vs. Eslicarbazepina: Ambas tienen mecanismos de acción similares, pero la eslicarbazepina se comercializa como un profármaco con una farmacocinética potencialmente mejorada.
This compound vs. Lamotrigina: Si bien ambas se utilizan para tratar la epilepsia, la lamotrigina también se utiliza para el trastorno bipolar y tiene un mecanismo de acción diferente que implica la inhibición de la liberación de glutamato.
La this compound destaca por su menor propensión a las interacciones medicamentosas y su eficacia en el control de las convulsiones de inicio parcial con un perfil de efectos secundarios relativamente favorable .
Aplicaciones Científicas De Investigación
Antiepileptic Use
Oxcarbazepine is effective in treating partial-onset seizures and primary generalized tonic-clonic seizures . It functions by blocking voltage-dependent sodium channels, which reduces abnormal electrical activity in the brain. The drug is indicated for use as both monotherapy and adjunctive therapy in adults and children aged four years and older .
Efficacy Studies
- Clinical Trials : Numerous studies have demonstrated that oxcarbazepine has comparable efficacy to other antiepileptic drugs such as carbamazepine, valproate, and phenytoin, with advantages in terms of side effects and pharmacokinetics .
- Meta-Analysis : A meta-analysis indicated that oxcarbazepine could effectively decrease seizure frequency in patients with drug-resistant epilepsy when used as an add-on therapy .
Psychiatric Applications
Oxcarbazepine has been explored as a mood stabilizer for conditions such as bipolar disorder . Although not FDA-approved for this indication, it is used off-label with some success.
Case Studies
- A case report documented a 53-year-old male with schizoaffective disorder who developed hyponatremia during treatment with oxcarbazepine, highlighting both its psychiatric application and potential side effects .
- Another study observed significant improvements in symptoms of bipolar disorder when oxcarbazepine was administered, suggesting its utility in managing mood disorders .
Neuropathic Pain Management
While evidence supporting oxcarbazepine's effectiveness in treating neuropathic pain is limited, some studies have suggested it may provide relief for conditions such as trigeminal neuralgia.
Research Findings
- A review indicated that oxcarbazepine could be beneficial for neuropathic pain management; however, the overall evidence remains inconclusive due to low patient numbers and event rates in studies .
Oncological Applications
Recent research has identified oxcarbazepine as a potential pro-apoptotic agent in certain cancer cell lines, particularly those with IDH mutations.
Experimental Findings
- In vitro studies showed that oxcarbazepine could inhibit the growth of glioma stem cells, suggesting a dual role as an antiepileptic and an antineoplastic agent . The treated cells exhibited significant reductions in size and increased apoptosis rates.
Adverse Effects and Considerations
Despite its therapeutic benefits, oxcarbazepine is associated with several adverse effects:
Análisis Bioquímico
Biochemical Properties
Oxcarbazepine exerts its effects by interacting with various biomolecules in the body. The primary biochemical property of oxcarbazepine is its ability to inhibit voltage-gated sodium channels, which are essential for the propagation of action potentials in neurons. By blocking these channels, oxcarbazepine reduces the abnormal electrical activity in the brain that leads to seizures . Additionally, oxcarbazepine undergoes rapid and extensive metabolism to its active metabolite, 10-hydroxycarbazepine, through the action of cytosolic arylketone reductase .
Cellular Effects
Oxcarbazepine has significant effects on various types of cells and cellular processes. In neurons, oxcarbazepine inhibits the excessive firing of action potentials by blocking voltage-gated sodium channels. This action stabilizes hyperexcited neural membranes and reduces the frequency of seizures . Oxcarbazepine also influences cell signaling pathways, gene expression, and cellular metabolism. It has been shown to modulate the release of neurotransmitters, thereby affecting synaptic transmission and neuronal communication .
Molecular Mechanism
The molecular mechanism of oxcarbazepine involves its interaction with voltage-gated sodium channels. By binding to these channels, oxcarbazepine prevents the influx of sodium ions, which is necessary for the initiation and propagation of action potentials in neurons . This blockade of sodium channels reduces neuronal excitability and prevents the spread of abnormal electrical activity in the brain. Additionally, oxcarbazepine’s active metabolite, 10-hydroxycarbazepine, contributes to its anticonvulsant effects by further inhibiting sodium channels .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of oxcarbazepine have been studied over time to understand its stability, degradation, and long-term impact on cellular function. Oxcarbazepine is rapidly absorbed and metabolized to 10-hydroxycarbazepine, which has a longer half-life and provides sustained anticonvulsant effects . Studies have shown that oxcarbazepine maintains its efficacy over extended periods, with minimal degradation . Long-term treatment with oxcarbazepine has been associated with stable seizure control and minimal adverse effects on cellular function .
Dosage Effects in Animal Models
The effects of oxcarbazepine vary with different dosages in animal models. At therapeutic doses, oxcarbazepine effectively reduces seizure frequency without causing significant adverse effects . At higher doses, oxcarbazepine can lead to toxic effects, including sedation, ataxia, and hepatotoxicity . Animal studies have also shown that chronic administration of oxcarbazepine can result in the development of tolerance, necessitating dose adjustments to maintain its anticonvulsant efficacy .
Metabolic Pathways
Oxcarbazepine is primarily metabolized in the liver through the action of cytosolic arylketone reductase, which converts it to its active metabolite, 10-hydroxycarbazepine . This metabolite is further metabolized through glucuronidation and hydroxylation pathways . The metabolic pathways of oxcarbazepine are distinct from those of carbamazepine, resulting in reduced drug-drug interactions and a more favorable safety profile .
Transport and Distribution
After oral administration, oxcarbazepine is rapidly absorbed and distributed throughout the body . Its active metabolite, 10-hydroxycarbazepine, has a volume of distribution of 0.75 L/kg and is extensively bound to plasma proteins . Oxcarbazepine and its metabolites are primarily excreted through the kidneys . The transport and distribution of oxcarbazepine within cells and tissues are influenced by its interactions with transporters and binding proteins .
Subcellular Localization
Oxcarbazepine and its active metabolite, 10-hydroxycarbazepine, are localized within the cytosol of cells . The subcellular localization of oxcarbazepine is crucial for its activity, as it allows the compound to interact with voltage-gated sodium channels and exert its anticonvulsant effects . The targeting of oxcarbazepine to specific cellular compartments is facilitated by its chemical structure and post-translational modifications .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La oxcarbazepina se sintetiza a partir de la carbamazepina mediante una reacción química que implica la oxidación de la molécula de carbamazepina. El proceso suele implicar el uso de agentes oxidantes como el peróxido de hidrógeno o los perácidos. Las condiciones de reacción incluyen temperatura y pH controlados para garantizar la oxidación selectiva de la carbamazepina a this compound .
Métodos de producción industrial: En entornos industriales, la producción de this compound implica reacciones de oxidación a gran escala utilizando agentes oxidantes similares. El proceso se optimiza para obtener un alto rendimiento y pureza, a menudo involucrando múltiples pasos de purificación como la recristalización y la cromatografía para eliminar las impurezas y los subproductos .
Análisis De Reacciones Químicas
Tipos de reacciones: La oxcarbazepina sufre diversas reacciones químicas, que incluyen:
Oxidación: Conversión de this compound a su metabolito activo, 10,11-dihidro-10-hidroxicarbamazepina (MHD).
Reducción: Las reacciones de reducción pueden revertir la this compound a sus formas precursoras.
Sustitución: Las reacciones de sustitución pueden ocurrir en las posiciones de amida o aromática
Reactivos y condiciones comunes:
Agentes oxidantes: Peróxido de hidrógeno, perácidos.
Agentes reductores: Borohidruro de sodio, hidruro de litio y aluminio.
Reactivos de sustitución: Halógenos, agentes alquilantes
Productos principales:
10,11-Dihidro-10-hidroxicarbamazepina (MHD): El principal metabolito activo.
Diversos derivados sustituidos: Dependiendo de los reactivos utilizados en las reacciones de sustitución
Comparación Con Compuestos Similares
Carbamazepine: The parent compound from which oxcarbazepine is derived.
Eslicarbazepine: Another derivative with similar anticonvulsant properties.
Lamotrigine: An anticonvulsant with a different chemical structure but similar therapeutic uses .
Comparison:
Oxcarbazepine vs. Carbamazepine: Oxcarbazepine has a better side effect profile and fewer drug interactions compared to carbamazepine.
Oxcarbazepine vs. Eslicarbazepine: Both have similar mechanisms of action, but eslicarbazepine is marketed as a prodrug with potentially improved pharmacokinetics.
Oxcarbazepine vs. Lamotrigine: While both are used to treat epilepsy, lamotrigine is also used for bipolar disorder and has a different mechanism of action involving the inhibition of glutamate release.
Oxcarbazepine stands out due to its reduced propensity for drug-drug interactions and its effectiveness in managing partial-onset seizures with a relatively favorable side effect profile .
Actividad Biológica
Oxcarbazepine (OXC) is an anticonvulsant medication primarily used for the treatment of epilepsy. It is a derivative of carbamazepine and functions by stabilizing neuronal membranes and inhibiting repetitive neuronal firing. This article explores the biological activity of oxcarbazepine, focusing on its mechanisms of action, therapeutic efficacy, and emerging research findings.
Oxcarbazepine acts through several mechanisms:
- Sodium Channel Blockade : OXC inhibits voltage-gated sodium channels, which reduces the excitability of neurons. This action is crucial in preventing seizures by decreasing abnormal electrical activity in the brain .
- Potassium Conductance : The drug enhances potassium conductance, contributing to its anticonvulsant properties .
- Calcium Channel Modulation : OXC modulates voltage-activated calcium channels, which may play a secondary role in its efficacy against seizures .
- Neurotransmitter Effects : Although initially thought to inhibit glutamatergic activity, this effect has not been consistently replicated in vivo .
Efficacy in Epilepsy Treatment
Numerous studies have evaluated the effectiveness of oxcarbazepine in treating various seizure types:
- Clinical Trials : A double-blind trial comparing oxcarbazepine with phenytoin (PHT) found no significant differences in seizure frequency between the two drugs. However, OXC demonstrated better tolerability with fewer severe side effects .
- Monotherapy vs. Add-on Therapy : In a study involving children and adolescents, OXC was shown to be effective as both a first-line monotherapy and an add-on therapy for partial seizures (PS) and generalized tonic-clonic seizures (GTCS) with comparable efficacy to carbamazepine .
- Long-Term Outcomes : A six-month follow-up study indicated that patients on OXC had significant improvements in mood and anxiety scales (SAS and SDS), suggesting additional psychological benefits beyond seizure control .
Proapoptotic Effects in Cancer Research
Recent research has identified oxcarbazepine's potential role beyond epilepsy treatment. A study published in 2023 highlighted its proapoptotic effects on IDH-mutant glioma cells, showing that OXC significantly reduced cell viability and induced apoptosis in tumor spheroids. The treated spheroids were found to be 82% smaller than controls after 72 hours, indicating substantial growth inhibition .
Table 1: Summary of Biological Activities of Oxcarbazepine
Case Studies and Emerging Research
- Efficacy in Bipolar Disorder : A pilot study suggested that oxcarbazepine may help prevent impulsivity and depressive episodes in bipolar patients when used as an adjunctive therapy to lithium. The results indicated a lower relapse rate among those treated with OXC compared to placebo .
- Retinoprotective Properties : Preliminary findings suggest that oxcarbazepine may have retinoprotective effects, showing no cytotoxicity in retinal cells while promoting cell proliferation under certain conditions .
Propiedades
IUPAC Name |
5-oxo-6H-benzo[b][1]benzazepine-11-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C15H12N2O2/c16-15(19)17-12-7-3-1-5-10(12)9-14(18)11-6-2-4-8-13(11)17/h1-8H,9H2,(H2,16,19) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CTRLABGOLIVAIY-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1C2=CC=CC=C2N(C3=CC=CC=C3C1=O)C(=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C15H12N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0045703 | |
| Record name | Oxcarbazepine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0045703 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
252.27 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble in chloroform, dichloromethane, acetone, and methanol and practically insoluble in ethanol, ether, and water., 1.60e-01 g/L | |
| Record name | OXCARBAZEPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7524 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The exact mechanism through which oxcarbazepine and its active metaoblite, MHD, exert their anti-epileptic effects is unclear, but is thought to primarily involve the blockade of voltage-gated sodium channels. The opening and closing of sodium channels allows for the propagation of action potentials along neurons - in epilepsy, these action potentials can occur in excess of that required for normal function, and the repetitive and pathological firing of these action potentials leads to seizure activity. Both oxcarbazepine and MHD are thought to inhibit seizure activity by binding to the inactive state of voltage-gated sodium channels, thus prolonging the period in which the receptor is unavailable for action potential propagation. This helps to stabilize hyperexcited neuronal membranes, inhibit repetitive neuron firing, and prevent the spread of seizure activity within the CNS without affecting normal neuronal transmission. Increased potassium conductance and modulation of voltage-activated calcium channels is also thought to play a role in the anti-seizure activity of oxcarbazepine. Inhibition of glutamatergic activity was thought to contribute to oxcarbazepine's activity, but this effect could not be replicated _in vivo_., The pharmacological activity of Trileptal (oxcarbazepine) is primarily exerted through the 10-monohydroxy metabolite (MHD) of oxcarbazepine. The precise mechanism by which oxcarbazepine and MHD exert their antiseizure effect is unknown; however, in vitro electrophysiological studies indicate that they produce blockade of voltage-sensitive sodium channels, resulting in stabilization of hyperexcited neural membranes, inhibition of repetitive neuronal firing, and diminution of propagation of synaptic impulses. These actions are thought to be important in the prevention of seizure spread in the intact brain. In addition, increased potassium conductance and modulation of high-voltage activated calcium channels may contribute to the anticonvulsant effects of the drug. No significant interactions of oxcarbazepine or MHD with brain neurotransmitter or modulator receptor sites have been demonstrated. | |
| Record name | Oxcarbazepine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00776 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | OXCARBAZEPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7524 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from ethanol, White to faintly orange crystalline powder | |
CAS No. |
28721-07-5 | |
| Record name | Oxcarbazepine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=28721-07-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Oxcarbazepine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0028721075 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Oxcarbazepine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00776 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | OXCARBAZEPINE | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758693 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Oxcarbazepine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0045703 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Oxcarbazepine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.044.702 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | OXCARBAZEPINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/VZI5B1W380 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | OXCARBAZEPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7524 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
215-216 °C, 215.5 °C | |
| Record name | OXCARBAZEPINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7524 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Oxcarbazepine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014914 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details







Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details





Synthesis routes and methods V
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.














