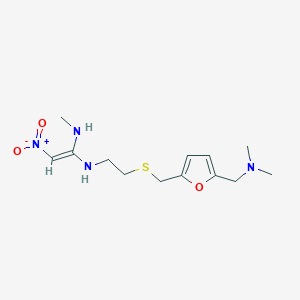
Ranitidine
Descripción general
Descripción
Ranitidina es un antagonista del receptor H2 de la histamina que se usó ampliamente para disminuir la producción de ácido estomacal. Se recetó comúnmente para el tratamiento de la enfermedad ulcerosa péptica, la enfermedad por reflujo gastroesofágico y el síndrome de Zollinger-Ellison. Ranitidina se descubrió en Inglaterra en 1976 y entró en uso comercial en 1981. Se comercializó con el nombre de marca Zantac, entre otros .
Mecanismo De Acción
Ranitidina funciona bloqueando los receptores H2 de la histamina en el revestimiento del estómago. La histamina, liberada de las células similares a las enterocromafines, se une a estos receptores y estimula la secreción de ácido gástrico. Al bloquear estos receptores, la ranitidina reduce la producción de ácido gástrico, aliviando así los síntomas asociados con el exceso de ácido estomacal .
Aplicaciones Científicas De Investigación
Gastrointestinal Disorders
1.1 Peptic Ulcer Disease
Ranitidine is primarily indicated for the treatment of peptic ulcers. Clinical studies have shown that it is effective in promoting healing of both gastric and duodenal ulcers. A meta-analysis indicated that this compound, at a dosage of 300 mg daily, is at least as effective as cimetidine in ulcer healing rates .
1.2 Gastroesophageal Reflux Disease (GERD)
In patients with GERD, this compound has demonstrated significant efficacy in reducing heartburn frequency and severity. A clinical trial involving 284 patients found that this compound treatment resulted in a marked decrease in heartburn symptoms compared to placebo, with improved endoscopic findings in patients with erosive esophagitis .
1.3 Zollinger-Ellison Syndrome
this compound is also utilized in the management of Zollinger-Ellison syndrome, a condition characterized by excessive gastric acid production. Studies confirm that this compound effectively reduces gastric acid secretion in these patients, thereby alleviating symptoms and preventing complications .
Surgical Applications
2.1 Prevention of Aspiration Pneumonitis
this compound has been used preoperatively to reduce the risk of aspiration pneumonitis during anesthesia induction. It is particularly administered to pregnant women and patients undergoing surgery to minimize gastric acidity and volume .
2.2 Stress Ulcer Prophylaxis
In critically ill patients, this compound is often prescribed for stress ulcer prophylaxis to prevent gastrointestinal bleeding due to stress-induced mucosal disease. Its role in this context remains under investigation but shows promise based on existing data .
Pediatric Use
This compound has been found safe for use in pediatric populations when dosed appropriately. It has been effective in treating conditions such as gastroesophageal reflux and peptic ulcers in children, with studies supporting its safety profile .
Analytical Studies and Validation
This compound's pharmacokinetics and efficacy have been extensively studied, leading to the establishment of validated analytical methods for its measurement in biological samples. These methods ensure accurate dosing and therapeutic monitoring .
Safety Considerations and Controversies
Recent concerns regarding the presence of N-nitrosodimethylamine (NDMA), a potential carcinogen, have led to increased scrutiny of this compound products. Regulatory agencies have issued recalls and advisories, prompting ongoing research into the long-term safety of this compound use .
Comparative Studies
A multinational cohort study investigated the cancer risk associated with this compound compared to other H2 receptor antagonists. The findings suggested no significant increase in cancer incidence among long-term users of this compound compared to those using alternatives like famotidine and lafutidine .
Data Summary Table
Análisis Bioquímico
Biochemical Properties
Ranitidine works by blocking the action of histamine on the H2 receptors of the parietal cells in the stomach, thereby reducing the production of stomach acid. The compound interacts with these receptors, preventing histamine from binding and triggering acid production .
Cellular Effects
This compound’s primary effect on cells is the reduction of gastric acid secretion in parietal cells. This can influence various cellular processes, including the regulation of intracellular pH and the activation of certain enzymes that require an acidic environment .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to H2 receptors on the parietal cells of the stomach. This prevents histamine from binding to these receptors and triggering the secretion of gastric acid. This action does not involve enzyme inhibition or activation, but rather receptor antagonism .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound are observed to be relatively stable over time. The drug does not undergo significant degradation and continues to exert its acid-suppressing effects as long as it is present in the system .
Dosage Effects in Animal Models
In animal models, the effects of this compound have been observed to be dose-dependent. Higher doses result in greater suppression of gastric acid secretion. Extremely high doses may lead to adverse effects, although these are generally rare .
Metabolic Pathways
This compound is metabolized in the liver through the cytochrome P450 system. It does not significantly interact with or alter other metabolic pathways .
Transport and Distribution
After oral administration, this compound is absorbed in the gastrointestinal tract and distributed throughout the body. It can cross cell membranes and reach its site of action in the stomach .
Subcellular Localization
This compound acts on the cell surface, specifically on the H2 receptors of parietal cells in the stomach. It does not have a specific subcellular localization as its site of action is on the cell surface .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La ranitidina se puede sintetizar a través de múltiples vías. Un método común involucra el intermedio 5-(dimetilamino)furfurylthioethylamine. La síntesis comienza con alcohol furfurilico, que se somete a una serie de reacciones para formar el intermedio. Este intermedio luego se hace reaccionar con l-metiltio-l-(N-metilamino)-2-nitroeteno para producir ranitidina .
Métodos de producción industrial: La producción industrial de ranitidina generalmente involucra el uso de solventes orgánicos y condiciones de reacción moderadas. Por ejemplo, el compuesto se puede sintetizar tratando un intermedio con sales de N,N-dimetilaminotrifenilfosfonio y dimetilamina a alrededor de 90 °C en solventes orgánicos como la dimetilformamida .
Análisis De Reacciones Químicas
Tipos de reacciones: Ranitidina experimenta diversas reacciones químicas, que incluyen:
Oxidación: Ranitidina se puede oxidar en ciertas condiciones, lo que lleva a la formación de diferentes subproductos.
Reactivos y condiciones comunes:
Oxidación: Se pueden usar agentes oxidantes comunes para oxidar ranitidina.
Fotólisis: Las reacciones de fotólisis generalmente requieren exposición a la luz y pueden verse influenciadas por la presencia de materia orgánica natural.
Principales productos formados:
Oxidación: Se pueden formar varios productos de oxidación, dependiendo de las condiciones y reactivos específicos utilizados.
Fotólisis: La fotólisis de ranitidina puede conducir a la formación de múltiples productos de degradación.
Comparación Con Compuestos Similares
Ranitidina pertenece a la clase de antagonistas del receptor H2 de la histamina, que también incluye compuestos como cimetidina y famotidina.
Compuestos similares:
Cimetidina: El primer antagonista del receptor H2 descubierto. Tiene un mecanismo de acción similar pero una estructura química diferente.
Singularidad de la ranitidina: Ranitidina se prefería a la cimetidina debido a su perfil de efectos secundarios mejorado y su potencia. Las preocupaciones sobre la presencia de N-nitrosodimetilamina en los productos de ranitidina han llevado a su retiro de muchos mercados .
Actividad Biológica
Ranitidine, a histamine H2 receptor antagonist, has been widely used for the treatment of various gastrointestinal disorders, including duodenal ulcers, gastric ulcers, gastroesophageal reflux disease (GERD), and Zollinger-Ellison syndrome. Its primary mechanism of action involves the reversible binding to H2 receptors on gastric parietal cells, leading to a reduction in gastric acid secretion. This article explores the biological activity of this compound, focusing on its pharmacological effects, potential risks associated with its use, and recent research findings.
This compound functions by blocking the action of histamine at the H2 receptors located on the gastric parietal cells. This interaction inhibits the secretion of gastric acid, providing relief from conditions associated with excess stomach acid. The onset of action typically occurs within 60 minutes after administration, with effects lasting up to 12 hours .
Pharmacological Effects
- Acid Secretion Inhibition : this compound significantly reduces gastric acid secretion, which is beneficial in treating acid-related disorders.
- Gastrin Levels : Prolonged use of this compound can lead to increased plasma gastrin levels due to feedback mechanisms triggered by reduced acidity .
- Neuronal Effects : Recent studies suggest this compound may have effects beyond acid inhibition, such as alleviating anxiety-like behaviors in animal models by enhancing exploratory locomotion and reducing anxiety-related behaviors .
Cancer Risk Studies
The safety profile of this compound has come under scrutiny due to concerns regarding its potential association with cancer risk:
- A multinational cohort study involving over 1.1 million individuals found no significant increase in cancer risk among new users of this compound compared to other H2 receptor antagonists (H2RAs). The crude incidence rates were similar, suggesting that this compound does not pose a higher risk for cancer than its counterparts .
- Conversely, another study indicated a statistically significant association between this compound use and an increased risk for certain gastrointestinal cancers (stomach and esophageal), with proportionate reporting ratios suggesting elevated risks for various cancers .
NDMA Contamination Concerns
One of the major concerns regarding this compound is its potential to form N-nitrosodimethylamine (NDMA), a probable human carcinogen:
- In vitro studies demonstrated that NDMA formation could occur under specific conditions involving high levels of nitrites in gastric fluid. However, rigorous clinical trials have shown no evidence of elevated NDMA levels in urine after administering this compound at standard doses .
- The FDA's studies indicated that this compound would only produce NDMA in simulated conditions far exceeding normal physiological levels of nitrite .
Case Studies
Several case studies have provided insights into the biological activity and safety profile of this compound:
- Study on Anxiety Reduction : In an experimental model using cysteamine HCl-treated mice, those treated with this compound exhibited significantly increased exploratory behavior compared to control groups. This suggests potential neuroactive properties beyond its gastrointestinal applications .
- Long-term Treatment Effects : A study conducted over 106 weeks in rats revealed that prolonged this compound treatment was associated with hyperplasia of gastric ECL cells and carcinoid tumors, raising concerns about long-term use in humans .
Summary Table: Key Findings on this compound
Propiedades
IUPAC Name |
(E)-1-N'-[2-[[5-[(dimethylamino)methyl]furan-2-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C13H22N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9,14-15H,6-8,10H2,1-3H3/b13-9+ | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VMXUWOKSQNHOCA-UKTHLTGXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CNC(=C[N+](=O)[O-])NCCSCC1=CC=C(O1)CN(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN/C(=C\[N+](=O)[O-])/NCCSCC1=CC=C(O1)CN(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C13H22N4O3S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID101112063 | |
| Record name | (1E)-N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101112063 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
314.41 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ranitidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001930 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Water soluble | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
H2 antagonists inhibit gastric acid secretion elicited by histamine and other H2 agonists in a dose dependent, competitive manner; the degree of inhibition parallels the concentration of the drug in plasma over a wide range. The H2 antagonists also inhibit acid secretion elicited by gastrin and, to a lesser extent, by muscarinic agonists. Importantly, these drugs inhibit basal (fasting) and nocturnal acid secretion and that stimulated by food, sham feeding, fundic distention, and various pharmacological agents; this property reflects the vital role of histamine in mediating the effects of diverse stimuli. /H2 Receptor Antagonists/, ... /H2 Antagonists/ measurably inhibit effects on the cardiovascular and other systems that are elicited through H2 receptors by exogenous or endogenous histamine. /H2 Receptor Antagonists/, ...IS A COMPETITIVE ANTAGONIST OF HISTAMINE-INDUCED GASTRIC ACID SECRETION... INHIBITS BOTH THE VOLUME AND CONCENTRATION OF GASTRIC ACID INDUCED NOCTURNALLY AND BY FOOD BUT DOES NOT AFFECT GASTRIC MUCUS OR ITS PRODUCTION. ...DOES NOT AFFECT LOWER ESOPHAGEAL SPHINCTER PRESSURE... | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
SOLID | |
CAS No. |
82530-72-1, 66357-35-5 | |
| Record name | (1E)-N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=82530-72-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | ranitidine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757851 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | (1E)-N-[2-[[[5-[(Dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N′-methyl-2-nitro-1,1-ethenediamine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101112063 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Ranitidine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.060.283 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ranitidine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001930 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
69-70 °C, MP: 133-134 °C /RATINIDINE HYDROCHLORIDE/ | |
| Record name | RANITIDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3925 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does ranitidine exert its therapeutic effect?
A: this compound acts as a competitive antagonist at histamine H2 receptors found on the basolateral membrane of parietal cells in the stomach. [] By blocking histamine binding to these receptors, this compound effectively reduces the secretion of gastric acid, providing relief from symptoms associated with hyperacidity. []
Q2: What are the key pharmacokinetic properties of this compound?
A: this compound is well absorbed after oral administration, reaching peak plasma concentrations within 1-3 hours. [] It is metabolized in the liver to several metabolites, with the primary metabolite being desmethylthis compound. [] Approximately 77% of an administered dose is excreted unchanged in the urine, with the remainder excreted as metabolites. [] The elimination half-life of this compound is 2.9-3.9 hours. []
Q3: Does this compound interact with other drugs?
A: Yes, this compound has been shown to interact with several drugs, primarily through its effects on drug-metabolizing enzymes in the liver. [] It can inhibit the cytochrome P450 enzyme system, particularly the CYP1A2 and CYP2D6 isoenzymes. [] This inhibition can lead to increased plasma concentrations of drugs that are metabolized by these enzymes, potentially resulting in adverse effects.
Q4: What are the safety concerns associated with this compound use?
A: While generally well-tolerated, this compound has been associated with rare but potentially serious adverse effects, including hypersensitivity reactions, hematological abnormalities, and hepatic dysfunction. [, ] Furthermore, the detection of N-nitrosodimethylamine (NDMA), a probable human carcinogen, in certain this compound formulations has raised concerns about potential long-term risks. []
Q5: What formulations of this compound are available?
A: this compound is available in various formulations, including oral tablets, effervescent tablets, syrups, and solutions for intravenous administration. [] The choice of formulation depends on the patient's age, medical condition, and preference.
Q6: What are the main therapeutic applications of this compound?
A6: this compound was widely prescribed for conditions associated with gastric hyperacidity, such as:
- Duodenal and gastric ulcers: Clinical trials demonstrated the efficacy of this compound in promoting ulcer healing and relieving symptoms. [, ]
- Gastroesophageal reflux disease (GERD): this compound effectively reduces heartburn and other symptoms of GERD. []
- Zollinger-Ellison syndrome: This rare condition involves excessive gastric acid production, and this compound can help manage symptoms. []
Q7: What alternatives to this compound are available for treating acid-related disorders?
A7: Several alternatives to this compound are available, including:
- Proton pump inhibitors (PPIs): These drugs, such as omeprazole, lansoprazole, and esomeprazole, are more potent inhibitors of gastric acid secretion than H2-receptor antagonists. []
- Antacids: These over-the-counter medications provide rapid but short-term relief from heartburn and indigestion by neutralizing stomach acid. []
- Alginates: These medications form a protective barrier over the stomach contents, preventing acid reflux into the esophagus. []
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.
















