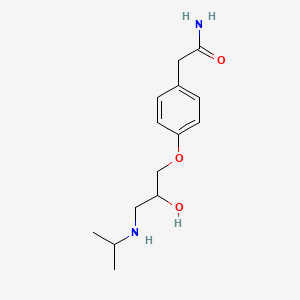
Atenolol
Descripción general
Descripción
Atenolol es un medicamento betabloqueante utilizado principalmente para tratar la presión arterial alta y el dolor en el pecho asociado al corazón. También se utiliza para prevenir las migrañas y tratar ciertos latidos cardíacos irregulares. This compound funciona bloqueando los receptores adrenérgicos beta-1 en el corazón, lo que disminuye la frecuencia cardíaca y la carga de trabajo .
Mecanismo De Acción
Atenolol funciona bloqueando selectivamente los receptores adrenérgicos beta-1 en el corazón. Estos receptores normalmente se unen a catecolaminas como la epinefrina y la norepinefrina, que aumentan la frecuencia cardíaca y la contractilidad. Al bloquear estos receptores, this compound reduce la frecuencia cardíaca, disminuye el gasto cardíaco y reduce la presión arterial. Este mecanismo ayuda a controlar afecciones como la hipertensión y la angina .
Compuestos Similares:
Metoprolol: Otro bloqueador selectivo beta-1 con usos similares pero diferentes propiedades farmacocinéticas.
Propranolol: Un betabloqueante no selectivo que afecta tanto a los receptores beta-1 como beta-2.
Labetalol: Un betabloqueante que también tiene propiedades de bloqueo alfa, utilizado para un control más completo de la presión arterial
Unicidad de this compound: this compound es único en su baja lipofilia, lo que reduce su capacidad de cruzar la barrera hematoencefálica. Esta propiedad da como resultado menos efectos secundarios en el sistema nervioso central en comparación con otros betabloqueantes como el propranolol .
Aplicaciones Científicas De Investigación
FDA-Approved Indications
Atenolol is approved for the following conditions:
- Hypertension : this compound effectively lowers blood pressure by reducing cardiac output and inhibiting sympathetic stimulation of the heart. Clinical studies have demonstrated significant reductions in blood pressure among patients treated with this compound compared to placebo .
- Angina Pectoris : The drug alleviates symptoms of angina by decreasing myocardial oxygen demand, thus improving exercise tolerance and reducing the frequency of angina attacks .
- Acute Myocardial Infarction : this compound is indicated for reducing mortality in patients with suspected or confirmed myocardial infarction by minimizing heart workload and oxygen consumption .
Off-Label Uses
In addition to its approved indications, this compound has several off-label applications:
- Arrhythmias : It is used to manage supraventricular and ventricular tachyarrhythmias due to its ability to prolong the refractory period of the atrioventricular node .
- Migraine Prophylaxis : this compound is recommended for preventing migraine attacks, as supported by guidelines from the American Academy of Neurology .
- Thyrotoxicosis Management : The drug can help control symptoms associated with hyperthyroidism, such as tachycardia .
- Alcohol Withdrawal : this compound is sometimes used in conjunction with benzodiazepines to manage symptoms during alcohol withdrawal .
Pharmacological Properties
This compound's mechanism of action involves selective inhibition of beta-1 adrenergic receptors, leading to decreased heart rate and myocardial contractility. This results in lowered blood pressure and reduced oxygen demand. Key pharmacokinetic properties include:
- Absorption : Approximately 50% bioavailability when taken orally; peak plasma levels occur within 2 to 4 hours .
- Distribution : Low lipid solubility limits its penetration into the central nervous system, resulting in fewer CNS-related side effects .
- Metabolism and Elimination : Minimal hepatic metabolism; primarily excreted via renal pathways with an elimination half-life of 6 to 7 hours .
Case Study 1: Hypertension Management
A double-blind trial involving 45 patients demonstrated that this compound significantly reduced blood pressure compared to placebo. The optimal daily dose for managing moderately severe hypertension was identified as 200 mg. Side effects were comparable between treatment groups, indicating a favorable safety profile .
Case Study 2: Migraine Prophylaxis
In a randomized controlled trial assessing this compound for migraine prevention, patients reported a notable decrease in the frequency of migraine attacks over a three-month period. This supports its use as an effective prophylactic treatment option for individuals suffering from episodic migraines .
Comparative Data Table
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción: Atenolol se sintetiza utilizando 3-(4-acetamido)fenoxi-1,2-epoxipropano e isopropilamina como materiales de partida. La reacción implica un proceso de aminación bajo la catálisis de tensioactivos gemini de éter carbónico. Este método es eficiente, produciendo this compound de alta pureza .
Métodos de Producción Industrial: La producción industrial de this compound sigue una ruta sintética similar, pero está optimizada para la producción a gran escala. El proceso implica el uso de disolventes eutécticos profundos, que permiten una síntesis sostenible y eficiente con un mínimo de residuos y un alto rendimiento .
Análisis De Reacciones Químicas
Tipos de Reacciones: Atenolol sufre varias reacciones químicas, incluyendo:
Oxidación: this compound puede oxidarse para formar diferentes metabolitos.
Reducción: Las reacciones de reducción son menos comunes, pero pueden ocurrir en condiciones específicas.
Sustitución: this compound puede sufrir reacciones de sustitución nucleofílica, particularmente involucrando el grupo hidroxilo.
Reactivos y Condiciones Comunes:
Oxidación: Los agentes oxidantes comunes incluyen peróxido de hidrógeno y permanganato de potasio.
Reducción: Se pueden utilizar agentes reductores como el borohidruro de sodio.
Sustitución: Los nucleófilos como las aminas y los tioles se utilizan comúnmente en reacciones de sustitución.
Productos Principales: Los productos principales formados a partir de estas reacciones incluyen varios metabolitos y derivados de this compound, que pueden tener diferentes propiedades farmacológicas .
Comparación Con Compuestos Similares
Metoprolol: Another beta-1 selective blocker with similar uses but different pharmacokinetic properties.
Propranolol: A non-selective beta-blocker that affects both beta-1 and beta-2 receptors.
Labetalol: A beta-blocker that also has alpha-blocking properties, used for more comprehensive blood pressure control
Uniqueness of Atenolol: this compound is unique in its low lipophilicity, which reduces its ability to cross the blood-brain barrier. This property results in fewer central nervous system side effects compared to other beta-blockers like propranolol .
Actividad Biológica
Atenolol is a selective beta-1 adrenergic antagonist primarily used in the treatment of hypertension and other cardiovascular conditions. Its biological activity extends beyond blood pressure regulation, influencing various metabolic and physiological processes. This article explores the biological mechanisms, therapeutic applications, and recent research findings associated with this compound.
This compound selectively binds to the β1-adrenergic receptors in cardiac tissues, which leads to a decrease in heart rate, myocardial contractility, and conduction velocity. This selectivity allows this compound to exert its effects primarily on the heart while minimizing interactions with β2 receptors found in the lungs and peripheral vasculature. The result is a reduction in cardiac workload and oxygen demand, which is beneficial for patients with hypertension and angina .
Pharmacokinetics
The pharmacokinetic profile of this compound includes:
- Absorption : Rapidly absorbed with peak plasma concentrations occurring within 2 to 4 hours post-administration.
- Distribution : Volume of distribution is approximately 4-5 L/kg, indicating extensive tissue uptake.
- Metabolism : Primarily excreted unchanged via the kidneys; minimal hepatic metabolism occurs.
- Half-life : Approximately 6 to 7 hours, allowing for once-daily dosing in many cases .
Therapeutic Uses
This compound's primary indications include:
- Hypertension : Proven efficacy in lowering blood pressure, with optimal doses reported around 100-200 mg daily for moderate hypertension .
- Angina Pectoris : Reduces frequency and severity of angina attacks by decreasing myocardial oxygen demand.
- Arrhythmias : Used to manage certain types of arrhythmias due to its effect on heart rate and conduction.
- Anxiety Disorders : Emerging evidence suggests this compound may be effective for anxiety management, with studies indicating positive responses among patients diagnosed with anxiety disorders .
Research Findings
Recent studies have expanded the understanding of this compound's biological activity:
- Impact on Metabolism : A study demonstrated that lifelong this compound treatment in mice improved mitochondrial function and reduced oxidative damage, suggesting potential benefits for metabolic health .
- Bone Health : Ongoing clinical trials are investigating this compound's role in preventing osteoporosis in postmenopausal women, focusing on its safety and effectiveness compared to standard treatments .
- Cancer Research : this compound has been shown to enhance the efficacy of metformin against breast cancer cells in preclinical models, indicating a potential role in cancer therapy through modulation of angiogenesis and tumor growth .
Case Study 1: Hypertension Management
In a controlled study involving 45 patients with essential hypertension, this compound was found to significantly reduce blood pressure compared to placebo. The study concluded that this compound is effective for managing hypertension with minimal side effects reported .
Case Study 2: Anxiety Disorders
In a preliminary investigation involving patients with anxiety disorders, 86% reported positive effects from this compound treatment. The majority found it preferable over other beta-blockers previously used for similar conditions .
Summary of Biological Effects
| Biological Effect | Description |
|---|---|
| Blood Pressure Reduction | Significant decrease in systolic and diastolic pressures |
| Heart Rate Control | Decreased resting heart rate and improved exercise tolerance |
| Metabolic Impact | Potential reduction in oxidative stress and improvement in mitochondrial function |
| Anxiety Reduction | Positive patient-reported outcomes in managing anxiety symptoms |
Propiedades
IUPAC Name |
2-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
METKIMKYRPQLGS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NCC(COC1=CC=C(C=C1)CC(=O)N)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H22N2O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID2022628 | |
| Record name | Atenolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022628 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
266.34 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Atenolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001924 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>40 [ug/mL] (The mean of the results at pH 7.4), Freely soluble in methanol; soluble in acetic acid, DMSO; sparingly soluble in 96% ethanol; slightly soluble in isopropanol; very slightly soluble in acetone, dioxane; practically insoluble in acetonitrile, ethyl acetate; chloroform, Freely soluble in 1 N HCl (300 mg/ml @ 25 °C); less soluble in chloroform (3 mg/ml @ 25 °C), In water, 1.33X10+4 mg/l @ 25 °C, 13.3 mg/mL at 25 °C | |
| Record name | SID855957 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Atenolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00335 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ATENOLOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6526 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Atenolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001924 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Atenolol is a cardioselective beta-blocker, called such because it selectively binds to the β1-adrenergic receptor as an antagonist up to a reported 26 fold more than β2 receptors. Selective activity at the β1 receptor produces cardioselectivity due to the higher population of this receptor in cardiac tissue. Some binding to β2 and possibly β3 receptors can still occur at therapeutic dosages but the effects mediated by antagonizing these are significantly reduced from those of non-selective agents. β1 and β2 receptors are Gs coupled therefore antagonism of their activation reduces activity of adenylyl cyclase and its downstream signalling via cyclic adenosime monophosphate and protein kinase A (PKA). In cardiomyocytes PKA is thought to mediate activation of L-type calcium channels and ryanodine receptors through their phosphorylation. L-type calcium channels can then provide an initial rise in intracellular calcium and trigger the ryanodine receptors to release calcium stored in the sarcoplasmic reticulum (SR) and increased contractility. PKA also plays a role in the cessation of contraction by phosphorylating phospholamban which in turn increases the affinity of SR Ca2+ ATPase to increase reuptake of calcium into the SR. It also phophorylates troponin I to reduce affinity of the protein for calcium. Both of these events lead to a reduction in contraction which, when coupled with the initial increase in contraction, allows for faster cycling and consequently higher heart rate with increased contractility. L-type calcium channels are also a major contributor to cardiac depolarization and their activation can increase frequency of action potentials and possibly the incidence of ectopic potentials. Similar inihibitory events occur in the bronchial smooth muscle to mediate relaxation including phosphorylation of myosin light-chain kinase, reducing its affinity for calcium. PKA also inhibits the excitatory Gq coupled pathway by phosphorylating the inositol trisphosphate receptor and phospholipase C resulting in inhibition of intracellular calcium release. Antagonism of this activity by beta-blocker agents like atenolol can thus cause increased bronchoconstriction., By inhibiting myocardial beta 1-adrenergic receptors, atenolol produces negative chronotropic and inotropic activity. The negative chronotropic action of atenolol on the sinoatrial node results in a decrease in the rate of sinoatrial node discharge and an increase in recovery time, thereby decreasing resting and exercise stimulated heart rate and reflex orthostatic tachycardia by about 25-35%. High doses of the drug may produce sinus arrest, especially in patients with sinoatrial node disease (eg, sick sinus syndrome). Atenolol also slows conduction in the atrioventricular nose. Although stroke index may be increased moderately by about 10%, atenolol usually reduces cardiac output by about 20% probably secondary to its effect on heart rate. The decrease in myocardial contractability and heart rate, as well as the reduction in blood pressure, produced by atenolol generally lead to a reduction in myocardial oxygen consumption which accounts for the effectiveness of the drug in chronic stable angina pectoris; however, atenolol can increase oxygen requirements by increasing left ventricular fiber length and end-diastolic pressure, particularly in patients with cardiac failure., Atenolol suppresses plasma renin activity and suppresses the renin aldosterone angiotensin system., The toxic actions of beta-blockers appear to be related to properties such as membrane depressant activity and possibly due to actions on beta-adrenoceptors distinct from those in the cardiovascular system. | |
| Record name | Atenolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00335 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ATENOLOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6526 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White, crystalline powder, Crystals from ethyl acetate, Colorless crystals | |
CAS No. |
29122-68-7 | |
| Record name | Atenolol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=29122-68-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Atenolol [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0029122687 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Atenolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00335 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | atenolol | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=757832 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Atenolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2022628 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Atenolol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.044.941 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ATENOLOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/50VV3VW0TI | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ATENOLOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6526 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Atenolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001924 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
158-160, 146 - 148 °C, 147 °C | |
| Record name | Atenolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00335 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ATENOLOL | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6526 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Atenolol | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001924 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.














