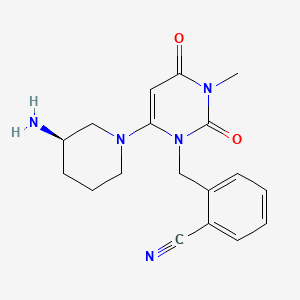
Alogliptin
Descripción general
Descripción
Alogliptina es un fármaco antidiabético oral que pertenece a la clase de inhibidores de la dipeptidil peptidasa-4 (DPP-4). Se utiliza principalmente para controlar la hiperglucemia en pacientes con diabetes mellitus tipo 2. Alogliptina funciona inhibiendo la enzima DPP-4, que normalmente degrada las hormonas incretinas como el polipéptido insulinotrópico dependiente de glucosa (GIP) y el péptido similar al glucagón-1 (GLP-1). Al inhibir la DPP-4, la alogliptina aumenta los niveles de hormonas incretinas activas, lo que mejora la secreción de insulina y reduce los niveles de glucagón de forma dependiente de la glucosa .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
La alogliptina se sintetiza mediante un proceso de varios pasos que implica la formación de intermedios clave. La ruta sintética suele comenzar con la preparación de 2-({6-[(3R)-3-aminopiperidin-1-il]-3-metil-2,4-dioxo-3,4-dihidropirimidin-1(2H)-il}metil)benzonitrilo. Este intermedio se somete entonces a diversas reacciones químicas, como la aminación y la ciclación, para obtener el producto final .
Métodos de producción industrial
En entornos industriales, la producción de alogliptina implica el uso de cromatografía líquida de alta resolución (HPLC) para la purificación y el control de calidad. El proceso se optimiza para garantizar un alto rendimiento y pureza del producto final. El uso de técnicas analíticas avanzadas, como la HPLC de fase inversa (RP-HPLC), es común en la producción industrial de alogliptina .
Análisis De Reacciones Químicas
Tipos de reacciones
La alogliptina se somete a diversas reacciones químicas, entre ellas:
Oxidación: La alogliptina puede oxidarse en condiciones específicas para formar los óxidos correspondientes.
Reducción: Las reacciones de reducción pueden utilizarse para modificar los grupos funcionales de la alogliptina.
Sustitución: La alogliptina puede someterse a reacciones de sustitución, en las que átomos o grupos específicos son sustituidos por otros
Reactivos y condiciones comunes
Los reactivos comunes utilizados en las reacciones químicas de la alogliptina incluyen:
Agentes oxidantes: Como el peróxido de hidrógeno y el permanganato de potasio.
Agentes reductores: Como el borohidruro de sodio y el hidruro de litio y aluminio.
Agentes sustituyentes: Como los halógenos y los agentes alquilantes
Principales productos formados
Los principales productos formados a partir de las reacciones químicas de la alogliptina dependen de las condiciones de reacción y los reactivos específicos utilizados. Por ejemplo, la oxidación puede producir óxidos, mientras que las reacciones de sustitución pueden dar lugar a derivados halogenados o alquilados .
Aplicaciones Científicas De Investigación
Management of Type 2 Diabetes Mellitus
Alogliptin is primarily indicated for improving glycemic control in adults with type 2 diabetes. It is often prescribed as monotherapy or in combination with other antidiabetic medications such as metformin or pioglitazone. Clinical trials have demonstrated that this compound effectively reduces hemoglobin A1c (HbA1c) levels, with studies showing a significant reduction of approximately 0.58% compared to placebo after 16 weeks .
Cardiovascular Safety and Outcomes
The EXAMINE trial evaluated the cardiovascular safety of this compound in patients with a recent acute coronary syndrome. Results indicated that this compound did not increase the risk of major adverse cardiovascular events compared to placebo, making it a viable option for diabetic patients with cardiovascular concerns . Additionally, long-term studies suggest that early initiation of this compound may prevent the progression of atherosclerosis in diabetic patients without prior cardiovascular disease .
Potential Renal Benefits
Research indicates that this compound may have protective effects on renal function in patients with type 2 diabetes. The drug has been associated with a lower incidence of renal impairment compared to other antidiabetic agents, suggesting its utility in managing diabetes-related kidney complications .
Efficacy and Safety Data
This compound has been shown to be effective in reducing fasting plasma glucose levels and achieving target HbA1c levels without causing significant weight gain or hypoglycemia, which are common concerns with other diabetes medications . The most frequent side effects reported include headaches, gastrointestinal disturbances, and skin rashes .
Case Study: Long-term Efficacy
In a cohort study involving 341 subjects, early initiation of this compound was linked to improved long-term cardiovascular outcomes without increasing cancer risk. This study highlights the potential for this compound not only to manage diabetes but also to contribute positively to overall patient health outcomes over extended periods .
Research Findings
- SPEAD-A Trial: This trial demonstrated that this compound significantly attenuated the progression of carotid atherosclerosis over two years, reinforcing its role in cardiovascular protection for diabetic patients .
- Phase 3 Trials: Multiple phase 3 trials have confirmed the efficacy and safety profile of this compound across diverse populations, including Asian cohorts, showing consistent results in HbA1c reduction and tolerability .
Mecanismo De Acción
La alogliptina ejerce sus efectos inhibiendo la enzima DPP-4, que es responsable de la degradación de las hormonas incretinas. Al inhibir la DPP-4, la alogliptina aumenta los niveles de hormonas incretinas activas, como el GLP-1 y el GIP. Estas hormonas mejoran la secreción de insulina por las células beta pancreáticas y reducen la secreción de glucagón por las células alfa pancreáticas, lo que lleva a una mejoría del control glucémico. La inhibición de la DPP-4 por la alogliptina también da lugar a niveles de incretina activos prolongados, lo que contribuye a sus efectos terapéuticos .
Comparación Con Compuestos Similares
La alogliptina forma parte de la clase de los inhibidores de la DPP-4, que incluye otros compuestos similares como:
- Sitagliptina
- Saxagliptina
- Linagliptina
- Vildagliptina
Singularidad de la alogliptina
La alogliptina es única entre los inhibidores de la DPP-4 debido a su alta selectividad y biodisponibilidad oral. Tiene una vida media relativamente larga, lo que permite una dosificación única al día. Además, se ha demostrado que la alogliptina tiene un perfil de seguridad favorable, con un bajo riesgo de hipoglucemia y un impacto mínimo en el peso corporal .
Actividad Biológica
Alogliptin is a selective dipeptidyl peptidase-4 (DPP-4) inhibitor utilized primarily in the management of type 2 diabetes mellitus (T2DM). Its mechanism of action involves the inhibition of DPP-4, an enzyme responsible for the degradation of incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By inhibiting DPP-4, this compound increases the levels of these hormones, leading to enhanced insulin secretion and improved glycemic control.
Pharmacokinetics and Pharmacodynamics
Absorption and Distribution:
this compound exhibits high bioavailability with a median time to peak concentration (Tmax) ranging from 1 to 2 hours and a half-life of approximately 12.4 to 21.4 hours. It is well-distributed in tissues, with a mean volume of distribution of 60.9 L, indicating extensive tissue penetration .
DPP-4 Inhibition:
Clinical studies show that this compound achieves significant DPP-4 inhibition, with mean plasma DPP-4 activity reduced by over 80% after 24 hours following administration. The inhibition rates after single doses range from 74.3% to 94% at 24 hours, and up to 99% after multiple doses over a period of 14 days .
Clinical Efficacy
This compound has been evaluated in various clinical trials for its efficacy in lowering hemoglobin A1c (HbA1c) levels and fasting plasma glucose (FPG).
Case Study Results
| Study Design | Population | Treatment Duration | HbA1c Reduction | FPG Reduction | Weight Change |
|---|---|---|---|---|---|
| Phase 3 Trial | 506 patients with T2DM | 16 weeks | -0.5% to -0.6% vs. placebo | Significant reduction (P ≤ 0.004) | No weight gain |
| Combination Therapy | Add-on to metformin or pioglitazone | 26 weeks | -0.8% with pioglitazone | Not specified | No significant change |
| Long-term Study | Randomized double-blind trial | 26 weeks | Modest improvement not statistically significant | Not specified | No significant change |
In a Phase 3 trial conducted across China, Taiwan, and Hong Kong, this compound significantly reduced HbA1c levels compared to placebo when used alone or in combination with other antidiabetic agents . The results indicated that patients receiving this compound were more likely to achieve target HbA1c levels of ≤6.5% or ≤7.0% compared to those on placebo.
Safety Profile
This compound is generally well-tolerated, with few adverse effects reported. The most common side effects include mild gastrointestinal disturbances and skin reactions such as pruritus. Importantly, no significant weight gain has been associated with its use, making it a favorable option for patients concerned about weight management .
Impact on Beta-cell Function
Research indicates that this compound may have a protective effect on pancreatic beta-cell function. However, studies have shown mixed results regarding its ability to improve beta-cell function metrics such as the proinsulin:insulin ratio and HOMA-β scores. While some studies suggest potential benefits, long-term data are required to confirm these findings .
Propiedades
IUPAC Name |
2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]benzonitrile | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H21N5O2/c1-21-17(24)9-16(22-8-4-7-15(20)12-22)23(18(21)25)11-14-6-3-2-5-13(14)10-19/h2-3,5-6,9,15H,4,7-8,11-12,20H2,1H3/t15-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZSBOMTDTBDDKMP-OAHLLOKOSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1C(=O)C=C(N(C1=O)CC2=CC=CC=C2C#N)N3CCCC(C3)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN1C(=O)C=C(N(C1=O)CC2=CC=CC=C2C#N)N3CCC[C@H](C3)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H21N5O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90234130 | |
| Record name | Alogliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90234130 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
339.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Sparingly soluble | |
| Record name | Alogliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06203 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Alogliptin inhibits dipeptidyl peptidase 4 (DPP-4), which normally degrades the incretins glucose-dependent insulinotropic polypeptide (GIP) and glucagon like peptide 1 ( GLP-1). The inhibition of DPP-4 increases the amount of active plasma incretins which helps with glycemic control. GIP and GLP-1 stimulate glucose dependent secretion of insulin in pancreatic beta cells. GLP-1 has the additional effects of suppressing glucose dependent glucagon secretion, inducing satiety, reducing food intake, and reducing gastric emptying., Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP-4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Alogliptin is a DPP-4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus. Alogliptin selectively binds to and inhibits DPP-4 but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures. | |
| Record name | Alogliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06203 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Alogliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8203 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
850649-61-5 | |
| Record name | Alogliptin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=850649-61-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Alogliptin [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0850649615 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Alogliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06203 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Alogliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90234130 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]benzonitrile | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ALOGLIPTIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/JHC049LO86 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Alogliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8203 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: [, , , , , , , , ] Alogliptin functions as a highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor. DPP-4 is an enzyme that rapidly inactivates incretin hormones, primarily glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By inhibiting DPP-4, this compound increases the levels of GLP-1 and GIP, which in turn leads to enhanced insulin secretion from pancreatic beta cells and suppressed glucagon secretion, ultimately resulting in improved glucose homeostasis.
A: The molecular formula of this compound benzoate (the active form) is C18H18N6O3 • C7H6O2, and its molecular weight is 462.46 g/mol.
A: While the provided papers don’t delve into detailed formulation strategies for this compound, one study explored the use of injectable long-acting poly (lactide-co-glycolide) (PLGA)-based in situ gel implants (ISGI) loaded with this compound . This approach aimed to provide sustained therapeutic exposures and improve pharmacological responses, potentially leading to reduced dosing frequency and enhanced patient compliance. The study highlighted the effectiveness of solvents like N-methyl-2-pyrrolidone (NMP) and dimethyl sulfoxide (DMSO) in achieving successful ISGI preparation and controlling the release profile of this compound.
A: [, , ] this compound demonstrates moderate absorption, exceeding 75%, and its absorption is not significantly affected by food [, ]. It exhibits slow-binding properties to the DPP-4 enzyme, resulting in sustained reduction of plasma DPP-4 activity . This compound has low plasma protein binding . Although the provided papers don't extensively detail its metabolism, they indicate that this compound is primarily excreted renally .
A: Research indicates that creatinine clearance and weight can significantly influence the oral clearance (CL/F) of this compound . This suggests that dose adjustments may be necessary for patients with renal impairment, while weight-based adjustments are generally not clinically relevant.
A: [, ] Studies haven't identified any significant drug interactions with this compound monotherapy [, ]. Notably, it can be co-administered with medications like ketoconazole, fluconazole, gemfibrozil, warfarin, metformin, glyburide, and pioglitazone without requiring dosage adjustments .
A: A study using diabetic apolipoprotein E-deficient mice showed that this compound treatment effectively reduced atherosclerotic lesions . This protective effect was linked to this compound's ability to lower plasma glucose levels and attenuate the expression of inflammatory cytokines (IL-6 and IL-1β) within the atherosclerotic plaques.
A: Research in rabbits suggests that this compound exhibits cardioprotective effects against ischemia-reperfusion injury . The study attributed these benefits to this compound's ability to enhance nitric oxide production through both GLP-1 receptor-dependent and -independent pathways. This increased nitric oxide production was associated with improved left ventricular ejection fraction and ±dP/dt, indicating enhanced cardiac function.
A: [, , , , , , , , , , ] Numerous clinical trials have demonstrated the efficacy of this compound in improving glycemic control in patients with type 2 diabetes. This compound, as monotherapy or in combination with other antidiabetic agents, consistently led to significant reductions in HbA1c levels [, , , , , , , , , , ]. Studies also highlight its efficacy in specific patient populations, such as those with inadequate glycemic control on metformin [, , ] or those receiving insulin therapy .
A: While traditional oral formulations of this compound are available, researchers are investigating injectable long-acting PLGA-based ISGI as a potential alternative delivery system. This approach aims to enhance patient compliance and potentially improve the therapeutic outcomes by achieving sustained drug release.
A: Research suggests that urinary angiotensinogen (AGT) levels could potentially serve as a prognostic marker for the renoprotective effects of this compound in patients with type 2 diabetes . The study observed a correlation between higher baseline urinary AGT levels and a more pronounced decrease in urinary albumin-to-creatinine ratio (UACR) following this compound treatment. This finding implies that urinary AGT could potentially help identify patients who are more likely to benefit from the renoprotective effects of this compound.
A: [, , ] Several analytical methods have been developed and validated for the quantification of this compound, including:
- UV Spectrophotometry: This technique utilizes the absorbance of UV-visible light by this compound at specific wavelengths for its quantification. It's a simple and cost-effective method often used for routine analysis of this compound in pharmaceutical formulations. [, ]
- High-Performance Thin-Layer Chromatography (HPTLC): HPTLC is a versatile technique that separates and quantifies this compound based on its differential migration on a thin layer of adsorbent material.
- Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC): RP-HPLC offers high sensitivity and selectivity in separating and quantifying this compound in complex matrices like pharmaceutical formulations and biological samples. It's often coupled with UV detection for quantification. [, ]
- Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS): LC-MS/MS provides exceptional sensitivity and specificity for quantifying this compound in biological matrices, particularly at low concentrations. It's often the preferred method for pharmacokinetic studies.
A: [, , ] The development and validation of analytical methods for this compound quantification involve assessing key validation parameters as outlined by regulatory guidelines, such as the International Conference on Harmonisation (ICH) guidelines. These parameters include:
A: [, , , , ] Several alternatives to this compound are available for managing type 2 diabetes, including:
- Other DPP-4 Inhibitors: As mentioned earlier, other DPP-4 inhibitors like sitagliptin, vildagliptin, saxagliptin, and linagliptin offer similar mechanisms of action and comparable efficacy to this compound in terms of HbA1c reduction. [, , , , ]
- Metformin: Metformin is a first-line treatment for type 2 diabetes that improves insulin sensitivity and reduces hepatic glucose production. It is often used in combination with DPP-4 inhibitors or GLP-1 receptor agonists. [, ]
A: [, ] this compound received approval from the US Food and Drug Administration (FDA) for the treatment of type 2 diabetes in 2013. [, ]
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















