Clofazimina
Descripción general
Descripción
La clofazimina es un colorante riminofenazínico antimicrobiano altamente lipofílico que se usa principalmente en el tratamiento de la lepra. Se describió por primera vez en 1957 y es conocido por su color rojo brillante, que puede causar decoloración de la piel y los fluidos corporales de larga duración . La this compound se usa en combinación con otros agentes, como la dapsona, para tratar la lepra lepromatosa, incluidos los casos resistentes a la dapsona .
Mecanismo De Acción
La clofazimina funciona uniéndose a las bases de guanina del ADN bacteriano, bloqueando así la función de plantilla del ADN e inhibiendo la proliferación bacteriana . También aumenta la actividad de la fosfolipasa A2 bacteriana, lo que lleva a la liberación y acumulación de lisofosfolípidos, que son tóxicos e inhiben la proliferación bacteriana . Además, la this compound ejerce propiedades antiinflamatorias al suprimir la actividad de los linfocitos T .
Compuestos similares:
Dapsona: Otro agente antimicrobiano utilizado en el tratamiento de la lepra.
Rifampicina: Se usa en combinación con this compound para el tratamiento de la lepra y la tuberculosis.
Unicidad de la this compound: La this compound es única debido a sus propiedades antimicrobianas y antiinflamatorias duales, así como su capacidad para acumularse en la piel y los nervios, lo que la hace particularmente eficaz en el tratamiento de la lepra .
Aplicaciones Científicas De Investigación
Clofazimine is a riminophenazine dye with a history of use in treating various diseases, including leprosy, tuberculosis, and infections caused by mycobacteria . Recent studies have also explored its potential in treating other conditions, such as Huntington's disease and viral infections .
Scientific Research Applications
Antimicrobial Applications
- Leprosy: Clofazimine is approved by the FDA for leprosy treatment . The National Hansen’s Disease Program (NHDP) uses clofazimine as part of a treatment protocol for multibacillary leprosy .
- Tuberculosis: Clofazimine was initially developed as an anti-tuberculosis treatment . It has been re-purposed to treat multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant TB (XDR-TB) .
- Nontuberculous Mycobacterial Infections: Clofazimine is used in combination with other antimicrobials to treat Mycobacterium avium complex (MAC) and is considered a promising drug for treating nontuberculous mycobacterial pulmonary disease .
Antiviral Applications
- Coronaviruses: Clofazimine has demonstrated inhibitory activity against coronaviruses, including SARS-CoV-2 and MERS-CoV . It inhibits cell fusion mediated by the viral spike glycoprotein and the viral helicase activity. In a hamster model of SARS-CoV-2, clofazimine reduced viral loads in the lung and viral shedding in feces and alleviated inflammation associated with viral infection .
- COVID-19: Clofazimine, combined with remdesivir, shows antiviral synergy in vitro and in vivo and restricts viral shedding from the upper respiratory tract . It is considered an attractive clinical candidate for treating outpatients and, when combined with remdesivir, hospitalized patients with COVID-19 .
Other Applications
- Huntington's Disease: Clofazimine may be effective in treating Huntington’s disease by reducing the toxicity of polyQ proteins and restoring mitochondrial function .
- Anti-inflammatory Properties: Clofazimine has been used or investigated for use in neutrophilic dermatoses, severe pyoderma gangrenosa, disseminated granuloma annulare, idiopathic panniculitis nodularis, autoimmune diseases, and lymphocytic dermatoses such as discoid lupus erythematosus .
- Anticancer Properties: Clofazimine has shown anticancer properties in vitro and in vivo, with its activity as a specific inhibitor of the oncogenic Wnt signaling pathway emerging as a promising targeting mechanism against breast, colon, liver, and other forms of cancer .
Research Findings
- Antiviral Mechanism: Transcriptional analysis revealed that clofazimine treatment could reverse transcriptomic changes induced by SARS-CoV-2 infection and prime innate-immunity-related pathways .
- In vivo Efficacy: Studies using a hamster model showed that clofazimine reduces body weight loss after SARS-CoV-2 infection, decreases virus plaque-forming units in lung tissues, and lowers viral loads in feces .
- Drug Repositioning: Clofazimine's journey from an anti-tuberculosis treatment to a leprosy drug and its subsequent repositioning for other infections and conditions exemplifies drug repurposing in modern pharmacology .
Case Studies
Due to the limitations of the provided search results, comprehensive case studies are not available. However, the following points highlight the clinical use and study of clofazimine in specific contexts:
- Expanded Access Programs: Real-world data collection from expanded access case studies has been conducted for treating nontuberculous mycobacterial infections with clofazimine .
- MDR-TB Treatment: Clofazimine's efficacy has been demonstrated as part of a shorter regimen for MDR-TB .
- MAC Pulmonary Disease: A study involving 170 patients with severe MAC-PD, treated with regimens containing clofazimine, suggests it as a promising drug .
Análisis Bioquímico
Biochemical Properties
Clofazimine exerts a slow bactericidal effect on Mycobacterium leprae due to its action on the bacterial outer membrane . There is also evidence that it affects the bacterial respiratory chain and ion transporters . Clofazimine is at least partially metabolized in the liver . An in vitro study using human liver microsomes identified eight metabolites of clofazimine and the enzymatic pathways involved in their formation, including the important cytochrome P450 isoenzymes CYP3A4/A5 and CYP1A2 .
Cellular Effects
Clofazimine has been found to modulate the expression of lipid metabolism proteins in Mycobacterium leprae-infected macrophages . It also exerts anti-inflammatory properties due to the suppression of T-lymphocyte activity . In HepaRG cells, clofazimine was a weak inducer of CYP3A4 at low concentrations, but inhibited CYP3A4 at therapeutic concentrations .
Molecular Mechanism
Clofazimine works by binding to the guanine bases of bacterial DNA, thereby blocking the template function of the DNA and inhibiting bacterial proliferation . It also increases the activity of bacterial phospholipase A2, leading to the release and accumulation of lysophospholipids, which are toxic and inhibit bacterial proliferation .
Temporal Effects in Laboratory Settings
In a study of patients with severe Mycobacterium avium complex pulmonary disease (MAC-PD), clofazimine demonstrated a relatively favorable efficacy, regardless of the maintenance dose . This effect was more pronounced when administered for a duration exceeding 6 months . In mice receiving clofazimine, the lungs’ bacterial load continued to grow during the first seven days of treatment .
Dosage Effects in Animal Models
In an orthotopic melanoma mouse model, clofazimine reduced tumor size by 90% . The specific effects of different dosages of clofazimine in animal models have not been extensively studied.
Metabolic Pathways
Clofazimine is involved in several metabolic pathways. It has been found to modulate the expression of lipid metabolism proteins in Mycobacterium leprae-infected macrophages . It also affects the bacterial respiratory chain and ion transporters .
Transport and Distribution
Clofazimine is a potential substrate of uptake and efflux transporters that might be involved in its disposition . The intracellular concentrations of clofazimine were significantly increased in the presence of selective inhibitors of P-gp and BCRP .
Subcellular Localization
Clofazimine has been found to accumulate in macrophages in an intracellular liquid crystal-like structure This suggests that clofazimine may be localized in specific subcellular compartments within these cells
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La clofazimina se sintetiza a través de un proceso de varios pasos que implica la condensación de 3-cloro-4-nitroanilina con 4-clorobenzaldehído para formar una base de Schiff. Este intermedio se cicla luego para formar la estructura central de fenazina. El paso final implica la reducción del grupo nitro a una amina .
Métodos de producción industrial: La producción industrial de this compound implica la homogeneización a alta presión para producir nanosuspensiones adecuadas para uso intravenoso. Este método asegura que el tamaño de partícula sea apropiado para la focalización pasiva al sistema reticuloendotelial .
Análisis De Reacciones Químicas
Tipos de reacciones: La clofazimina experimenta varias reacciones químicas, incluyendo oxidación, reducción y sustitución. Se sabe que interactúa con los fosfolípidos de membrana, lo que lleva a la generación de lisofosfolípidos antimicrobianos .
Reactivos y condiciones comunes: Los reactivos comunes utilizados en la síntesis y reacciones de la this compound incluyen 3-cloro-4-nitroanilina, 4-clorobenzaldehído y agentes reductores para el paso final .
Productos principales: El producto principal formado a partir de la síntesis de this compound es la estructura central de riminofenazina, que es esencial para su actividad antimicrobiana .
Comparación Con Compuestos Similares
Dapsone: Another antimicrobial agent used in the treatment of leprosy.
Rifampin: Used in combination with clofazimine for the treatment of leprosy and tuberculosis.
Uniqueness of Clofazimine: Clofazimine is unique due to its dual antimicrobial and anti-inflammatory properties, as well as its ability to accumulate in skin and nerves, making it particularly effective in treating leprosy .
Actividad Biológica
Clofazimine, a riminophenazine dye, was originally developed for the treatment of leprosy and has since gained recognition for its efficacy against multidrug-resistant tuberculosis (MDR-TB) and other mycobacterial infections. This article delves into the biological activity of clofazimine, highlighting its pharmacokinetics, antimicrobial properties, and clinical outcomes based on diverse research findings.
Pharmacokinetics
Clofazimine exhibits unique pharmacokinetic properties characterized by its long half-life , which can extend from weeks to months depending on the duration of administration. The drug accumulates in tissues, achieving concentrations sometimes up to milligrams per gram, while serum levels remain relatively low and steady. This accumulation allows for sustained antimicrobial activity even after treatment cessation, with significant declines in colony-forming unit (CFU) counts observed in various studies .
Table 1: Pharmacokinetic Parameters of Clofazimine
| Parameter | Value |
|---|---|
| Half-life | Weeks to months |
| Tissue concentration | Up to mg/g |
| Serum concentration | ~1.0 µg/ml (at 25 mg/kg) |
Antimicrobial Activity
Clofazimine demonstrates delayed antimicrobial activity against Mycobacterium tuberculosis both in vitro and in vivo. Studies have shown that it significantly reduces CFU counts in the lungs and spleens of infected mice. For instance, a study reported a decline of 2.4 log CFU in lungs after 8 weeks of treatment with clofazimine alone . Additionally, when combined with other anti-TB drugs, clofazimine enhances overall treatment efficacy.
Case Study: Efficacy Against Non-Tuberculous Mycobacteria (NTM)
A recent study involving 170 patients with severe Mycobacterium avium complex pulmonary disease (MAC-PD) treated with clofazimine-containing regimens revealed promising results. The microbiological cure rate reached 71% when clofazimine was administered for 6-12 months , compared to just 23% for those treated for less than 6 months . This underscores the importance of treatment duration in achieving successful outcomes.
Clinical Outcomes
Clofazimine's clinical effectiveness has been evaluated through various cohort studies. A systematic review indicated that approximately 65% of patients treated with clofazimine-containing regimens experienced favorable outcomes, such as cure or treatment completion . Notably, it has shown effectiveness even in cases of extensively drug-resistant tuberculosis (XDR-TB), although higher mortality rates have been observed compared to other treatments .
Table 2: Treatment Outcomes in Clofazimine Studies
| Study Type | Total Patients | Culture Conversion (%) | Microbiological Cure (%) |
|---|---|---|---|
| Cohort Study on MAC-PD | 170 | 45.3 | 54.6 |
| Systematic Review (MDR-TB) | Various | N/A | 65 |
Side Effects and Safety Profile
Clofazimine is generally well-tolerated; however, it is associated with certain side effects such as gastrointestinal complaints and skin hyperpigmentation. In one analysis, only 11.4% of patients reported adverse events related to clofazimine, leading to discontinuation in less than 1% of cases . This safety profile is crucial for long-term treatments often required for MDR-TB.
Propiedades
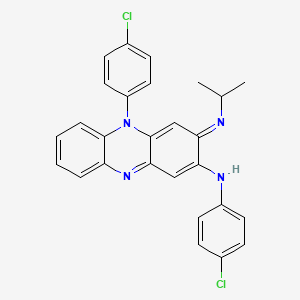
IUPAC Name |
N,5-bis(4-chlorophenyl)-3-propan-2-yliminophenazin-2-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H22Cl2N4/c1-17(2)30-24-16-27-25(15-23(24)31-20-11-7-18(28)8-12-20)32-22-5-3-4-6-26(22)33(27)21-13-9-19(29)10-14-21/h3-17,31H,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WDQPAMHFFCXSNU-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)N=C1C=C2C(=NC3=CC=CC=C3N2C4=CC=C(C=C4)Cl)C=C1NC5=CC=C(C=C5)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H22Cl2N4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7022839 | |
| Record name | Clofazimine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022839 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
473.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
0.3 [ug/mL] (The mean of the results at pH 7.4), 1.51e-03 g/L | |
| Record name | SID49681815 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Although the precise mechanism(s) of action of clofazimine have not been elucidated, its antimicrobial activity appears to be membrane-directed. It was previously thought that, due to its lipophilicity, clofazimine participated in the generation of intracellular reactive oxygen species (ROS) via redox cycling, specifically H2O2 and superoxide, which then exerted an antimicrobial effect. A more recent and compelling theory involves clofazimine interacting with bacterial membrane phospholipids to generate antimicrobial lysophospholipids - bactericidal efficacy may, then, arise from the combined membrane-destabilizing effects of both clofazimine and lysophospholipids, which interfere with K+ uptake and, ultimately, ATP production. The anti-inflammatory activity of clofazimine is the result of its inhibition of T-lymphocyte activation and proliferation. Several mechanisms have been proposed, including direct antagonism of T-cell Kv 1.3 potassium channels and indirect action by promoting the release of E-series prostaglandins and reactive oxygen species from bystander neutrophils and monocytes. | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
2030-63-9 | |
| Record name | Clofazimine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=2030-63-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Clofazimine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0002030639 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | clofazimine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759283 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | clofazimine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=141046 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Clofazimine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022839 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Clofazimine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.016.347 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLOFAZIMINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/D959AE5USF | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
210-212 °C, 210 - 212 °C | |
| Record name | Clofazimine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00845 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Clofazimine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014983 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















