Linagliptin
Descripción general
Descripción
Linagliptina es un medicamento utilizado para tratar la diabetes mellitus tipo 2. Es un inhibidor de la dipeptidil peptidasa-4 (DPP-4) que funciona aumentando la producción de insulina y disminuyendo la producción de glucagón por el páncreas . Linagliptina se administra por vía oral y se comercializa bajo los nombres comerciales Tradjenta, Trajenta y Trazenta .
Métodos De Preparación
Linagliptina se sintetiza mediante una serie de reacciones químicas que involucran compuestos intermedios. Un proceso mejorado para preparar Linagliptina implica purificar los compuestos intermedios y convertirlos en Linagliptina . La síntesis normalmente incluye pasos como reacciones de condensación, purificación y cristalización para lograr un alto rendimiento y pureza . Los métodos de producción industrial se centran en optimizar estos pasos para garantizar una calidad y eficiencia consistentes .
Análisis De Reacciones Químicas
Linagliptina experimenta diversas reacciones químicas, que incluyen oxidación, reducción y sustitución. Los reactivos comunes utilizados en estas reacciones incluyen agentes oxidantes, agentes reductores y catalizadores . Por ejemplo, Linagliptina es particularmente susceptible a la degradación cuando se expone a ácido y peróxido, lo que lleva a la formación de productos de degradación . Comprender estas reacciones es crucial para optimizar su formulación y garantizar su eficacia terapéutica .
Aplicaciones Científicas De Investigación
Management of Type 2 Diabetes Mellitus
Linagliptin is primarily indicated for the treatment of type 2 diabetes. It can be used as monotherapy or in combination with other antidiabetic agents. Clinical trials have demonstrated that this compound effectively lowers hemoglobin A1c levels with a low risk of hypoglycemia.
- Efficacy Comparison : In a study comparing this compound to glimepiride, both medications showed similar reductions in hemoglobin A1c, but this compound had a significantly lower incidence of hypoglycemia and cardiovascular events .
| Study | Treatment | HbA1c Reduction | Hypoglycemia Incidence |
|---|---|---|---|
| This compound vs Glimepiride | -0.16% | 1% | |
| This compound vs Glimepiride | -0.36% | 2% |
Cardiovascular Benefits
Recent studies suggest that this compound may offer cardiovascular protection. It has been associated with improved endothelial function and reduced cardiovascular events in patients with type 2 diabetes.
- Endothelial Function Improvement : A randomized study showed that after 16 weeks of treatment with this compound, patients exhibited significant improvements in flow-mediated dilation, indicating enhanced endothelial function .
Renal Protection
This compound has shown nephroprotective effects in preclinical models and clinical settings. It mitigates kidney fibrosis and improves albuminuria without altering glucose levels.
- Mechanisms : The protective effects are attributed to the suppression of pro-inflammatory cytokines and oxidative stress pathways .
| Study | Outcome | Findings |
|---|---|---|
| Kidney Health | Reduced kidney fibrosis and albuminuria in animal models | |
| In Vitro Studies | Inhibition of TGF-β activation |
Potential Use in COVID-19 Management
A clinical trial investigated the efficacy of this compound in hospitalized patients with type 2 diabetes and COVID-19. Although no significant difference was found compared to standard care, the study highlighted the need for further research into its immunomodulatory effects .
Case Study 1: Cardiovascular Outcomes
A long-term follow-up study involving over 3000 participants assessed the impact of this compound on major adverse cardiovascular events. The results indicated a lower incidence of cardiovascular complications compared to traditional therapies .
Case Study 2: Renal Health Impact
In a cohort study focusing on diabetic patients with renal impairment, this compound treatment was associated with significant reductions in markers of kidney damage, suggesting its role as a protective agent against diabetic nephropathy .
Mecanismo De Acción
Linagliptina ejerce sus efectos inhibiendo la enzima DPP-4, que es responsable de la degradación de las hormonas incretinas como el péptido similar al glucagón 1 (GLP-1) y el polipéptido insulinotrópico dependiente de la glucosa (GIP) . Al inhibir la DPP-4, Linagliptina aumenta los niveles de hormonas incretinas activas, lo que lleva a un aumento de la producción de insulina y una disminución de la producción de glucagón . Esto ayuda a regular los niveles de glucosa en sangre en pacientes con diabetes tipo 2 .
Comparación Con Compuestos Similares
Linagliptina es uno de los varios inhibidores de la DPP-4 que se utilizan para tratar la diabetes tipo 2. Otros compuestos similares incluyen sitagliptina, saxagliptina y alogliptina . En comparación con estos compuestos, Linagliptina tiene un perfil farmacocinético único, con una larga vida media terminal y eliminación predominantemente no renal . Esto permite la dosificación una vez al día sin necesidad de ajuste de la dosis en pacientes con insuficiencia renal . Además, Linagliptina ha mostrado una inhibición sostenida y máxima de la actividad de la DPP-4, que no se observa con algunos otros inhibidores de la DPP-4 .
Actividad Biológica
Linagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor primarily used in the management of type 2 diabetes mellitus. It has gained attention not only for its efficacy in glycemic control but also for its potential biological activities beyond glucose regulation. This article explores the biological activity of this compound, including its pharmacodynamics, pharmacokinetics, and emerging therapeutic roles.
Pharmacodynamics
Mechanism of Action
this compound selectively inhibits DPP-4, an enzyme that degrades incretin hormones, which are critical for insulin secretion and glucose homeostasis. The compound exhibits a potent inhibition profile with an IC50 value of 1 nM, outperforming other DPP-4 inhibitors like sitagliptin and saxagliptin . this compound's high selectivity for DPP-4 (over 10,000-fold compared to other dipeptidyl peptidases) enhances its therapeutic profile by minimizing off-target effects .
Biological Effects
Research indicates that this compound may exert antioxidant effects due to its xanthine-based structure, which is significant in reducing oxidative stress in various tissues . In experimental models of autoimmune myocarditis, this compound treatment resulted in a marked reduction in inflammatory cell infiltration and myocardial damage .
Table 1: Comparison of DPP-4 Inhibitors
| Compound | IC50 (nM) | Selectivity for DPP-4 |
|---|---|---|
| This compound | 1 | >10,000-fold |
| Sitagliptin | 19 | Not specified |
| Alogliptin | 24 | Not specified |
| Saxagliptin | 50 | Not specified |
| Vildagliptin | 62 | Not specified |
Pharmacokinetics
This compound demonstrates unique pharmacokinetic properties characterized by extensive tissue distribution and a nonlinear increase in tissue concentrations with rising doses. Following administration, this compound binds to DPP-4 primarily in tissues such as the kidney, liver, and lung . Its absorption is influenced by intestinal P-glycoprotein, indicating a complex interaction with gastrointestinal physiology .
Table 2: Pharmacokinetic Parameters of this compound
| Parameter | Value |
|---|---|
| Bioavailability | High |
| Half-life | ~12 hours |
| Volume of distribution | High |
| Tissue binding | Significant in DPP-4 rich tissues |
Case Study: this compound in COVID-19 Patients
A randomized clinical trial assessed the efficacy of this compound compared to standard care in hospitalized patients with diabetes and COVID-19. The study involved 64 participants who received either this compound (5 mg daily) or standard therapy. Although there was no significant difference in the time to clinical improvement between groups (7 days for this compound vs. 8 days for standard care), this compound was associated with lower in-hospital mortality rates .
Table 3: Clinical Outcomes from the COVID-19 Study
| Outcome | This compound Group (n=32) | Standard Care Group (n=32) |
|---|---|---|
| Median Time to Improvement | 7 days (IQR: 3.5–15) | 8 days (IQR: 3.5–28) |
| In-hospital Mortality | 15.6% | 25.0% |
Emerging Therapeutic Roles
Recent studies suggest potential applications of this compound beyond diabetes management. Its anti-inflammatory properties may be beneficial in conditions like autoimmune diseases and cardiovascular disorders due to its ability to modulate immune responses and reduce oxidative stress .
Q & A
Basic Research Questions
Q. What experimental models are most validated for assessing Linagliptin’s mechanism of action in type 2 diabetes?
- Methodological Answer : Begin with in vitro dipeptidyl peptidase-4 (DPP-4) inhibition assays using recombinant enzymes or cell lines (e.g., Caco-2 cells) to quantify inhibitory concentration (IC50). Validate findings in rodent models (e.g., streptozotocin-induced diabetic mice) with endpoints like HbA1c reduction and glucose tolerance tests. Ensure consistency with human trials by cross-referencing pharmacokinetic parameters (e.g., bioavailability, half-life) .
Q. How should researchers standardize purity assessments for this compound in preclinical studies?
- Methodological Answer : Employ high-performance liquid chromatography (HPLC) with UV detection (λ = 210–230 nm) and mass spectrometry (MS) for structural confirmation. For novel formulations, include nuclear magnetic resonance (NMR) spectroscopy and elemental analysis. Purity thresholds (>98%) should align with International Council for Harmonisation (ICH) guidelines, with batch-to-batch variability documented in supplementary materials .
Q. What statistical approaches are recommended for analyzing this compound’s dose-response relationships?
- Methodological Answer : Use non-linear regression models (e.g., sigmoidal Emax) to estimate EC50 and maximal efficacy. For clinical data, apply mixed-effects models to account for inter-individual variability. Sensitivity analyses should adjust for covariates like renal impairment, which affects this compound’s clearance .
Advanced Research Questions
Q. How can conflicting data on this compound’s cardiorenal outcomes be reconciled across trials?
- Methodological Answer : Conduct a meta-analysis stratified by patient subgroups (e.g., baseline renal function, cardiovascular risk profiles). Use propensity score matching to balance covariates. For mechanistic insights, integrate transcriptomic data (e.g., RNA sequencing of kidney tissues) to identify pathways modulated by this compound beyond DPP-4 inhibition .
Q. What strategies address the limited long-term safety data for this compound in elderly populations?
- Methodological Answer : Design prospective cohort studies with extended follow-up (≥5 years), leveraging real-world data from electronic health records. Apply competing risk models to differentiate drug-related adverse events (e.g., pancreatitis) from age-associated comorbidities. Validate findings using in vitro senescence models to study drug toxicity in aged cells .
Q. How do researchers optimize experimental designs to study this compound’s off-target effects?
- Methodological Answer : Utilize high-throughput screening (e.g., kinase profiling panels) to identify off-target interactions. Validate hits using CRISPR/Cas9-mediated gene knockout in relevant cell lines. For in vivo relevance, employ transgenic models (e.g., DPP-4 knockout mice) to isolate this compound-specific effects from endogenous enzyme activity .
Q. What methodologies elucidate this compound’s interaction with gut microbiota in metabolic outcomes?
- Methodological Answer : Perform 16S rRNA sequencing of fecal samples from clinical cohorts, correlating microbial diversity with glycemic responses. Use germ-free mice colonized with human microbiota to test causal relationships. Metabolomic profiling (e.g., LC-MS) can identify microbial-derived metabolites (e.g., short-chain fatty acids) modulated by this compound .
Q. Data and Reproducibility
Q. How should researchers handle variability in this compound’s pharmacokinetic data across ethnic populations?
- Methodological Answer : Implement population pharmacokinetic (PopPK) modeling to assess ethnic differences in drug metabolism. Include covariates like CYP3A4/5 polymorphisms and body mass index (BMI). Validate models using bootstrap or visual predictive checks. Share raw data via repositories (e.g., ClinicalTrials.gov ) to enhance reproducibility .
Q. What frameworks guide the integration of contradictory findings into a cohesive mechanistic model for this compound?
- Methodological Answer : Apply systems biology approaches (e.g., network pharmacology) to map this compound’s interactions across proteomic and metabolomic datasets. Use Bayesian statistics to weigh evidence from conflicting studies. Publish negative results and methodological limitations transparently to refine hypotheses .
Q. Ethical and Reporting Standards
Q. How can preclinical studies on this compound adhere to NIH guidelines for rigor and reproducibility?
- Methodological Answer : Follow ARRIVE 2.0 guidelines for animal studies, including randomization, blinding, and sample size justification. For in vitro work, document cell line authentication (e.g., STR profiling) and mycoplasma testing. Pre-register protocols on platforms like Open Science Framework to mitigate bias .
Propiedades
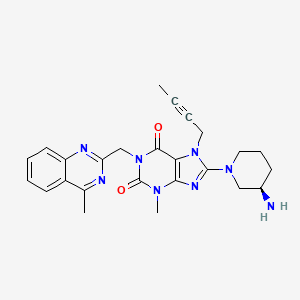
IUPAC Name |
8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]purine-2,6-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LTXREWYXXSTFRX-QGZVFWFLSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC#CCN1C2=C(N=C1N3CCCC(C3)N)N(C(=O)N(C2=O)CC4=NC5=CC=CC=C5C(=N4)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC#CCN1C2=C(N=C1N3CCC[C@H](C3)N)N(C(=O)N(C2=O)CC4=NC5=CC=CC=C5C(=N4)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C25H28N8O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID201021653 | |
| Record name | Linagliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201021653 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
472.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
<1 mg/mL, Soluble in methanol; sparingly soluble in ethanol; very slightly soluble in isopropanol, alcohol | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Linagliptin is a competitive, reversible DPP-4 inhibitor. Inhibition of this enzyme slows the breakdown of GLP-1 and glucose-dependant insulinotropic polypeptide (GIP). GLP-1 and GIP stimulate the release of insulin from beta cells in the pancreas while inhibiting release of glucagon from pancreatic beta cells. These effects together reduce the breakdown of glycogen in the liver and increase insulin release in response to glucose., Linagliptin is an inhibitor of DPP-4, an enzyme that degrades the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Thus, linagliptin increases the concentrations of active incretin hormones, stimulating the release of insulin in a glucose-dependent manner and decreasing the levels of glucagon in the circulation. Both incretin hormones are involved in the physiological regulation of glucose homeostasis. Incretin hormones are secreted at a low basal level throughout the day and levels rise immediately after meal intake. GLP-1 and GIP increase insulin biosynthesis and secretion from pancreatic beta-cells in the presence of normal and elevated blood glucose levels. Furthermore, GLP-1 also reduces glucagon secretion from pancreatic alpha-cells, resulting in a reduction in hepatic glucose output. | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to yellow solid; also reported as a crystalline solid | |
CAS No. |
668270-12-0 | |
| Record name | Linagliptin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=668270-12-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Linagliptin [USAN:INN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0668270120 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID201021653 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2yl)methyl]purine-2,6-dione | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LINAGLIPTIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3X29ZEJ4R2 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
190-196, 202 °C | |
| Record name | Linagliptin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08882 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Linagliptin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8204 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















