Estavudina
Descripción general
Descripción
Stavudine es un inhibidor de la transcriptasa inversa análogo de nucleósido (ITIAN) utilizado principalmente en el tratamiento de la infección por el virus de la inmunodeficiencia humana (VIH). Fue descrito por primera vez en 1966 y aprobado para su uso en los Estados Unidos en 1994 . Stavudine funciona inhibiendo la actividad de la transcriptasa inversa del VIH-1, una enzima crucial para la replicación del virus .
Mecanismo De Acción
Stavudine inhibe la actividad de la transcriptasa inversa del VIH-1 compitiendo con el sustrato natural desoxiguanosina trifosfato (dGTP) e incorporándose al ADN viral. Esta incorporación da como resultado la terminación de la síntesis de ADN, lo que evita que el virus se replique . Stavudine se fosforila a metabolitos activos que compiten por la incorporación al ADN viral, inhibiendo la enzima de forma competitiva .
Aplicaciones Científicas De Investigación
Clinical Efficacy
Stavudine Monotherapy vs. Zidovudine
A randomized controlled trial involving 822 HIV-infected adults compared stavudine monotherapy with zidovudine. Results indicated that patients receiving stavudine had a lower rate of clinical progression (26 per 100 person-years) compared to those on zidovudine (32 per 100 person-years), demonstrating a relative risk reduction of 25% for clinical endpoints in the stavudine group (P = 0.03) . The study highlighted the drug's effectiveness across different CD4+ cell strata and clinical stages of HIV disease.
Combination Therapy
In combination therapies, stavudine has been evaluated alongside other antiretroviral agents. A notable trial compared the efficacy of stavudine combined with lamivudine and efavirenz against tenofovir DF with similar combinations. The findings suggested that both regimens effectively reduced HIV RNA levels to below 400 copies/mL, although tenofovir showed a more favorable safety profile .
Pharmacokinetics and Dosage Adjustments
Dose Reduction Studies
Research has indicated that reducing the dose of stavudine can mitigate some adverse effects while maintaining viral suppression. A study demonstrated that switching from a standard 40 mg dose to a reduced 30 mg dose improved mitochondrial function indicators and decreased serum lactate levels without compromising HIV control . This finding is crucial for managing metabolic toxicities often associated with NRTIs.
Adverse Effects and Management
Neuropathy and Metabolic Toxicity
One of the significant concerns with stavudine use is its association with peripheral neuropathy, which occurred in 12% of patients compared to only 4% in those receiving zidovudine . Additionally, metabolic side effects such as lactic acidosis and lipodystrophy have been reported. Strategies to mitigate these effects include careful monitoring and dose adjustments.
Comparative Effectiveness
Phasing Out Stavudine
Recent trends indicate a shift away from stavudine towards newer agents like tenofovir due to concerns regarding toxicity. A study showed that patients on tenofovir had significantly lower rates of drug substitution due to adverse effects compared to those on stavudine . This highlights the ongoing evolution in HIV treatment protocols as newer therapies become available.
Case Studies
- ALBI Trial : This trial assessed the combination of stavudine and didanosine against zidovudine and lamivudine over 24 weeks. The results indicated superior reductions in HIV-1 RNA levels in the stavudine group, reinforcing its efficacy as part of combination therapy .
- Longitudinal Observational Studies : Observations from various cohorts indicated that while stavudine was effective initially, long-term use led to increased rates of side effects, prompting clinicians to consider alternatives like tenofovir or zidovudine .
Análisis Bioquímico
Biochemical Properties
Stavudine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1) . It is phosphorylated to active metabolites that compete for incorporation into viral DNA . These metabolites inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis .
Cellular Effects
Stavudine influences cell function by inhibiting the activity of HIV-1 reverse transcriptase (RT), an enzyme crucial for the replication of HIV . By competing with the natural substrate dGTP and by its incorporation into viral DNA, Stavudine prevents the formation of the 5’ to 3’ phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated .
Molecular Mechanism
Stavudine exerts its effects at the molecular level by inhibiting the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA . The lack of a 3’-OH group in the incorporated nucleoside analogue prevents the formation of the 5’ to 3’ phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated .
Metabolic Pathways
Stavudine is involved in the metabolic pathway of HIV replication. It interacts with the enzyme HIV-1 reverse transcriptase (RT) to inhibit the replication of the virus .
Métodos De Preparación
Rutas de Síntesis y Condiciones de Reacción: Stavudine se puede sintetizar mediante varios métodos. Un método común implica la conversión de timidina a stavudine a través de una serie de reacciones químicas. El proceso generalmente incluye el uso de reactivos como trifosgeno y piridina, seguido de pasos de desprotección para producir el producto final .
Métodos de Producción Industrial: En entornos industriales, la stavudine se produce utilizando técnicas de síntesis química a gran escala. El proceso implica múltiples pasos, incluida la protección de grupos funcionales, reacciones selectivas para introducir las modificaciones deseadas y pasos de purificación para obtener stavudine de alta pureza .
Análisis De Reacciones Químicas
Tipos de Reacciones: Stavudine experimenta diversas reacciones químicas, incluyendo:
Oxidación: Stavudine puede oxidarse para formar diferentes metabolitos.
Reducción: Las reacciones de reducción pueden modificar los grupos funcionales en stavudine.
Sustitución: Las reacciones de sustitución pueden introducir nuevos grupos funcionales en la molécula de stavudine.
Reactivos y Condiciones Comunes:
Oxidación: Los agentes oxidantes comunes incluyen permanganato de potasio y peróxido de hidrógeno.
Reducción: Se utilizan agentes reductores como el borohidruro de sodio.
Sustitución: Se emplean reactivos como haluros de alquilo y nucleófilos.
Principales Productos Formados: Los principales productos formados a partir de estas reacciones incluyen varios metabolitos y derivados modificados de stavudine, que pueden tener diferentes propiedades farmacológicas .
Comparación Con Compuestos Similares
Stavudine es similar a otros inhibidores de la transcriptasa inversa análogos de nucleósidos como la didanosina y la zalcitabina. tiene propiedades únicas que lo distinguen de estos compuestos:
Didanosina: También un ITIAN, pero difiere en su estructura química y farmacocinética.
Zalcitabina: Otro ITIAN con un mecanismo de acción y un perfil de efectos secundarios diferentes.
Lista de Compuestos Similares:
- Didanosina
- Zalcitabina
- Zidovudina
La estructura química y el mecanismo de acción únicos de Stavudine lo convierten en un compuesto valioso en el tratamiento de la infección por VIH y un tema de investigación científica continua.
Actividad Biológica
Stavudine, also known as d4T, is a nucleoside reverse transcriptase inhibitor (NRTI) primarily used in the treatment of HIV-1 infection. Its mechanism of action, pharmacokinetics, and associated toxicities have been extensively studied, revealing significant insights into its biological activity.
Stavudine is phosphorylated within cells to active metabolites that competitively inhibit the HIV-1 reverse transcriptase enzyme. This inhibition occurs through two primary mechanisms:
- Competitive Inhibition : Stavudine competes with the natural substrate deoxyguanosine triphosphate (dGTP) for incorporation into viral DNA.
- Chain Termination : Once incorporated, stavudine lacks a 3'-OH group, preventing the formation of the essential 5' to 3' phosphodiester linkage required for DNA chain elongation. This results in termination of viral DNA synthesis .
Pharmacokinetics
Stavudine exhibits rapid absorption following oral administration, with bioavailability ranging from 68% to 104% . It is primarily eliminated through renal clearance and hepatic metabolism, with approximately 40% excreted unchanged in urine . The pharmacokinetic profile suggests that stavudine can be effectively combined with other antiretroviral agents without significant drug interactions .
Efficacy and Dosage
Research has shown that a reduced dosage of stavudine (30 mg twice daily) can maintain efficacy comparable to the standard dose (40 mg twice daily) while reducing adverse effects such as mitochondrial toxicity and bone mineral density loss . A systematic review indicated that lower doses could preserve virological suppression while improving mitochondrial indices in patients .
Table 1: Comparison of Stavudine Dosages and Outcomes
| Dosage (mg) | Efficacy | Mitochondrial Toxicity | Bone Mineral Density Loss |
|---|---|---|---|
| 40 mg | High | Significant | Significant |
| 30 mg | Equivalent | Reduced | Minimal |
Toxicity Profile
Stavudine is associated with several toxicities, including:
- Peripheral Neuropathy : A common side effect, often dose-dependent.
- Hyperlactatemia : Increased lactate levels can lead to lactic acidosis.
- Mitochondrial Toxicity : Changes in mitochondrial DNA content have been observed, particularly in adipose tissue and muscle .
A longitudinal study found that reducing the stavudine dose led to improvements in mitochondrial function, as evidenced by increased fat mtDNA and decreased lactate levels while maintaining HIV suppression .
Case Studies
- Longitudinal Study on Stavudine Toxicity : This study tracked a cohort over several years, identifying peripheral neuropathy as a predominant toxicity. Patients who switched to lower doses reported fewer side effects without loss of virological control .
- Comparative Study with Abacavir : A retrospective analysis compared treatment outcomes in HIV-infected children receiving either stavudine or abacavir. The results demonstrated comparable efficacy but highlighted differences in toxicity profiles, with stavudine showing higher rates of peripheral neuropathy .
Propiedades
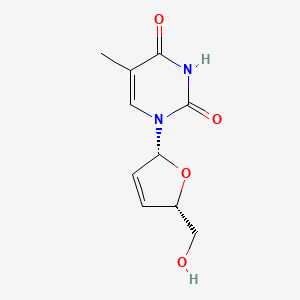
IUPAC Name |
1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XNKLLVCARDGLGL-JGVFFNPUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=CN(C(=O)NC1=O)C2C=CC(O2)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=CN(C(=O)NC1=O)[C@H]2C=C[C@H](O2)CO | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H12N2O4 | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID1023819 | |
| Record name | Stavudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1023819 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
224.21 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
2',3'-didehydro-3'-deoxythymidine appears as white crystalline solid or powder. Odorless. (NTP, 1992), Solid | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
50 to 100 mg/mL at 70 °F (NTP, 1992), 5-10 g/100 mL at 21 °C, 30 mg/mL in propylene glycol at 23 °C, In water, 83 mg/mL at 23 °C, 4.05e+01 g/L | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
9.5X10-12 mm Hg at 25 °C /Estimated/ | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Stavudine inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA., Enzymatic conversion of stavudine to d4T-triphosphate appears to be complex, involving several steps and enzymes. Stavudine is first converted to dideoxydidehydrothymidine-5'-monophosphate (d4T-monophosphate) by thymidine kinase. Subsequently, d4T-monophosphate is converted to dideoxydidehydrothymidine-5'-diphosphate (d4T-diphosphate), and then to d4T-triphosphate, presumably by the same cellular kinases involved in the metabolism of zidovudine. ... d4T-Triphosphate is a structural analog of thymidine triphosphate, the natural substrate for viral RNA-directed DNA polymerase. ... d4T-triphosphate appears to compete with thymidine triphosphate for viral RNA-directed DNA polymerase and incorporation into viral DNA. Following incorporation of d4T-triphosphate into the viral DNA chain instead of thymidine triphosphate, synthesis is terminated prematurely because the absence of the 3'-hydroxy group on the drug prevents further 5' to 3' phosphodiester linkages., Stavudine is phosphorylated by cellular kinases to the active metabolite stavudine triphosphate. Stavudine triphosphate inhibits the activity of HIV reverse transcriptase both by competing with the natural substrate deoxythymidine triphosphate (Ki =0.0083 to 0.032 uM), and by its incorporation into viral DNA causing a termination of DNA chain elongation because stavudine lacks the essential 3'-OH group. Stavudine triphosphate inhibits cellular DNA polymerase beta and gamma, and markedly reduces the synthesis of mitochondrial DNA., d4T-Triphosphate can bind to and inhibit some mammalian cellular DNA polymerases, particularly beta- and gamma-polymerases, in vitro, and markedly reduce the synthesis of mitochondrial DNA. ...gamma-polymerase, an enzyme involved in mitochondrial DNA synthesis, is the polymerase most susceptible to inhibition. However, d4T-triphosphate and other dideoxynucleoside triphosphates appear to have much greater affinity for viral RNA-directed DNA polymerase than for mammalian DNA polymerases. ... Inhibition of beta- and gamma-polymerases by these drugs may account, to some extent, for the toxic effects associated with stavudine and other nucleosides in humans. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to off white crystalline solid, Colorless granular solid from ethanol/benzene | |
CAS No. |
3056-17-5 | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=3056-17-5 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Stavudine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0003056175 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | stavudine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759897 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Stavudine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID1023819 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 1-((2R, 5S)-5-(hydroxymethyl)-2,5-dihydro-2-furanyl)-5-methyl-2,4(1H, 3H)-pyrimidinedione | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | STAVUDINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/BO9LE4QFZF | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
318 to 320 °F (NTP, 1992), 159-160 °C, 165-166 °C, 159 - 160 °C | |
| Record name | 2',3'-DIDEHYDRO-3'-DEOXYTHYMIDINE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20175 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Stavudine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00649 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | STAVUDINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7338 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Stavudine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014787 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















