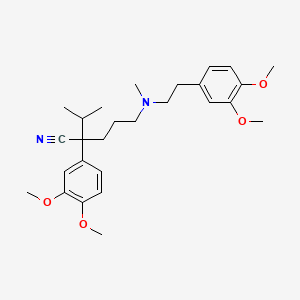
Verapamilo
Descripción general
Descripción
El verapamilo es un bloqueador de los canales de calcio de tipo fenilalquilamina que se utiliza principalmente en el tratamiento de afecciones cardiovasculares como la presión arterial alta, la angina de pecho y ciertos tipos de arritmias . Fue el primer antagonista de los canales de calcio que se introdujo en la terapia a principios de la década de 1960 . El this compound está disponible en diversas formulaciones, incluidas la oral y la intravenosa, y es ampliamente reconocido por su eficacia en el control de la frecuencia cardíaca y la mejora del flujo sanguíneo .
Mecanismo De Acción
El verapamilo ejerce sus efectos al inhibir la entrada de iones calcio a través de los canales lentos en las membranas celulares del músculo liso arterial y las células miocárdicas . Esta inhibición conduce a la relajación de los vasos sanguíneos, la reducción de la frecuencia cardíaca y la disminución de la demanda de oxígeno del miocardio . Los objetivos moleculares del this compound incluyen los canales de calcio de tipo L, que desempeñan un papel fundamental en la función cardiovascular .
Aplicaciones Científicas De Investigación
Cardiovascular Applications
Mechanism of Action
Verapamil primarily functions by inhibiting calcium influx through L-type calcium channels, which leads to vasodilation and reduced myocardial contractility. This mechanism underlies its efficacy in treating various cardiovascular conditions.
Therapeutic Uses
- Atrial Tachyarrhythmias : Verapamil is effective in managing paroxysmal supraventricular tachycardia and atrial fibrillation by slowing conduction through the atrioventricular node .
- Angina Pectoris : It alleviates angina symptoms by reducing myocardial oxygen demand through vasodilation and decreased heart rate .
- Hypertension : Clinical studies have demonstrated significant reductions in both systolic and diastolic blood pressure with verapamil treatment over extended periods .
Clinical Evidence
A study published in PubMed reported that after two years of treatment with verapamil, patients exhibited a reduction in blood pressure by approximately 16.3% systolic and 16.6% diastolic .
Diabetes Management
Recent research has unveiled verapamil's potential in treating Type 1 diabetes.
Case Study: Type 1 Diabetes Clinical Trials
- Study Overview : A randomized, double-blind, placebo-controlled trial conducted by the University of Alabama at Birmingham found that oral administration of verapamil significantly preserved beta-cell function in patients with newly diagnosed Type 1 diabetes .
- Results : Participants receiving verapamil showed a 30% improvement in insulin secretion compared to the control group over one year . This finding suggests that verapamil may enhance endogenous insulin production, reducing reliance on exogenous insulin injections.
The implications of these findings are profound, as they indicate a potential shift in how Type 1 diabetes may be managed therapeutically.
Cancer Treatment
Verapamil is also being investigated for its role in oncology, particularly regarding its ability to enhance the efficacy of chemotherapeutic agents.
Mechanism in Cancer Therapy
- Inhibition of Drug Resistance : Research indicates that verapamil can inhibit P-glycoprotein, a transporter associated with multidrug resistance in tumor cells. By blocking this transporter, verapamil enhances the effectiveness of anticancer drugs like paclitaxel .
- Combination Therapy : A study demonstrated that combining low-dose paclitaxel with verapamil improved anti-tumor effects and reduced metastasis in animal models .
Summary Table of Applications
Análisis Bioquímico
Biochemical Properties
Verapamil interacts with various enzymes and proteins, primarily through its role as a calcium channel blocker. It is known to interact with cytochrome P450 enzymes, which are involved in its metabolism . Verapamil is also a potent inhibitor of P-glycoprotein function , which plays a crucial role in drug transport and distribution.
Cellular Effects
Verapamil has been shown to have significant effects on various types of cells and cellular processes. It influences cell function by blocking calcium channels, which can impact cell signaling pathways, gene expression, and cellular metabolism . For instance, verapamil has been found to regulate the thioredoxin system and promote an anti-oxidative, anti-apoptotic, and immunomodulatory gene expression profile in human islets .
Molecular Mechanism
Verapamil exerts its effects at the molecular level primarily through its role as a calcium channel blocker. It binds to L-type calcium channels, inhibiting the influx of calcium ions into cells . This can lead to a decrease in intracellular calcium levels, which can affect various cellular processes, including muscle contraction, cell signaling, and neurotransmitter release.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of verapamil can change over time. For example, a study showed that long-term low-dose verapamil effectively prevents the development of cognitive impairment induced by a certain compound in aged animals . This suggests that verapamil may have long-term effects on cellular function in both in vitro and in vivo studies.
Dosage Effects in Animal Models
The effects of verapamil can vary with different dosages in animal models. For instance, a study showed that verapamil at a dose of 1mg/kg/d prevented the development of cognitive impairment in an aged mouse model of sporadic Alzheimer’s disease .
Metabolic Pathways
Verapamil is involved in various metabolic pathways. It is subject to extensive oxidative metabolism mediated by cytochrome P450 enzymes, with less than 5% of an oral dose being excreted unchanged in urine . Verapamil is also known to be a potent inhibitor of P-glycoprotein function, which can affect the transport and distribution of various other drugs .
Transport and Distribution
Verapamil is widely distributed throughout body tissues . It is transported and distributed within cells and tissues primarily through its interaction with P-glycoprotein, a transporter protein that plays a crucial role in drug transport and distribution .
Subcellular Localization
A study investigating the transport of fluorescence-labeled verapamil at the blood-retinal barrier suggested that verapamil may be partially sequestered in lysosomes within retinal pigment epithelial cells .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
El verapamilo se sintetiza a través de un proceso de varios pasos que implica la reacción de 3,4-dimetoxi-feniletilamina con cloruro de isovalerilo para formar un intermedio, que luego se hace reaccionar con 3,4-dimetoxi-bencilocianuro en condiciones específicas . El producto final se purifica para lograr un alto grado de pureza, a menudo superior al 99% .
Métodos de producción industrial
La producción industrial de this compound normalmente implica síntesis a gran escala utilizando vías de reacción similares a las de los entornos de laboratorio, pero optimizadas para la eficiencia y la rentabilidad. Se emplean técnicas como las resinas de intercambio iónico y los métodos de preparación de liposomas para mejorar la biodisponibilidad y la liberación controlada del fármaco .
Análisis de las reacciones químicas
Tipos de reacciones
El this compound se somete a diversas reacciones químicas, incluidas la oxidación, la reducción y la sustitución. Estas reacciones son esenciales para su metabolismo y farmacocinética .
Reactivos y condiciones comunes
Los reactivos comunes que se utilizan en las reacciones que involucran this compound incluyen agentes oxidantes como el ozono y los radicales hidroxilo . Las condiciones para estas reacciones se controlan cuidadosamente para garantizar los resultados deseados, como la formación de metabolitos específicos.
Principales productos formados
Los principales productos formados a partir de las reacciones del this compound incluyen sus metabolitos, que se excretan principalmente a través de los riñones . Estos metabolitos son cruciales para comprender los efectos farmacológicos del fármaco y los posibles efectos secundarios.
Análisis De Reacciones Químicas
Types of Reactions
Verapamil undergoes various chemical reactions, including oxidation, reduction, and substitution. These reactions are essential for its metabolism and pharmacokinetics .
Common Reagents and Conditions
Common reagents used in the reactions involving verapamil include oxidizing agents like ozone and hydroxyl radicals . The conditions for these reactions are carefully controlled to ensure the desired outcomes, such as the formation of specific metabolites.
Major Products Formed
The major products formed from the reactions of verapamil include its metabolites, which are primarily excreted through the kidneys . These metabolites are crucial for understanding the drug’s pharmacological effects and potential side effects.
Comparación Con Compuestos Similares
Compuestos similares
El verapamilo pertenece a la clase de bloqueadores de los canales de calcio no dihidropiridínicos, que incluye otros compuestos como el diltiazem y la flunarizina . Estos compuestos comparten mecanismos de acción similares, pero difieren en sus estructuras químicas y perfiles farmacocinéticos.
Unicidad
El this compound es único en su capacidad de actuar como un agente antiarrítmico y antihipertensivo, lo que lo hace versátil en el tratamiento de diversas afecciones cardiovasculares . Su estructura química distintiva permite interacciones específicas con los canales de calcio, lo que proporciona un rango terapéutico más amplio en comparación con otros bloqueadores de los canales de calcio .
Actividad Biológica
Verapamil is a well-known L-type calcium channel blocker primarily used in the treatment of cardiovascular conditions such as hypertension, angina, and certain arrhythmias. However, recent research has expanded our understanding of its biological activity beyond cardiovascular effects, revealing potential applications in diabetes management, neuroprotection, and even anti-aging mechanisms.
Verapamil exerts its pharmacological effects by inhibiting calcium influx through L-type calcium channels, which leads to a decrease in intracellular calcium levels. This action results in vasodilation, reduced heart rate, and decreased myocardial contractility. Additionally, verapamil has been shown to influence various signaling pathways and gene expressions related to inflammation and oxidative stress.
Antiglycoxidant Activity
Recent studies have highlighted verapamil's antiglycoxidant properties . A study demonstrated that verapamil significantly reduced biomarkers of protein glycation and oxidation in vitro, comparable to recognized antiglycating agents. Molecular docking simulations indicated that verapamil preferentially binds to amino acids susceptible to glycoxidative damage, particularly interacting with Janus kinase 2 (JAK2) and nuclear factor-κB (NF-κB) pathways .
Impact on Pancreatic β-Cell Function
Verapamil has shown promising results in preserving pancreatic β-cell function in children with newly diagnosed Type 1 Diabetes (T1D). A multi-center clinical trial indicated that patients treated with verapamil exhibited elevated C-peptide levels, suggesting improved β-cell function. Importantly, no serious adverse events were reported during the study, although some psychiatric disorders were noted .
Neuroprotective Effects
The compound also demonstrates neuroprotective effects against neurodegenerative conditions. Research indicates that verapamil can restore microtubule-binding activity of tau protein and reduce oxidative stress in neuronal cells exposed to amyloid-beta peptides, which are implicated in Alzheimer's disease. These findings suggest a potential role for verapamil in neuroprotection and cognitive health .
Anti-Aging Properties
In model organisms like Caenorhabditis elegans, verapamil has been associated with lifespan extension by inhibiting calcineurin activity. This inhibition is linked to improved healthspan and cellular viability in mammalian cells, indicating its potential as an anti-aging compound .
Table 1: Summary of Clinical Studies on Verapamil's Biological Activity
Case Studies
- Antiglycoxidant Properties : In a controlled study evaluating the effect of verapamil on glycation processes, researchers found that treatment led to a significant decrease in advanced glycation end-products (AGEs), suggesting potential benefits for diabetic patients at risk for vascular complications .
- Type 1 Diabetes : A randomized trial involving pediatric patients with newly diagnosed T1D showed that those treated with verapamil had sustained improvements in β-cell function over time compared to controls. This study emphasizes the need for further research into the long-term benefits of calcium channel blockers in diabetes management .
- Neuroprotection : In preclinical studies using neuronal cell lines exposed to amyloid-beta toxicity, verapamil demonstrated protective effects by mitigating neurotoxic impacts and restoring normal cellular functions, indicating a potential therapeutic avenue for Alzheimer's disease .
Propiedades
IUPAC Name |
2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methylamino]-2-propan-2-ylpentanenitrile | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
SGTNSNPWRIOYBX-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(CCCN(C)CCC1=CC(=C(C=C1)OC)OC)(C#N)C2=CC(=C(C=C2)OC)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H38N2O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9041152 | |
| Record name | (+/-)-Verapamil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9041152 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
454.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Verapamil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001850 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
BP: 245 °C at 0.01 mm Hg, 243-246 °C at 1.00E-02 mm Hg | |
| Record name | Verapamil | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3928 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Verapamil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001850 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Solubility (mg/mL): water 83, ethanol (200 proof) 26, propylene glycol 93, ethanol (190 proof) >100, methanol >100, 2-propanol 4.6, ethyl acetate 1.0, DMF >100, methylene chloride >100, hexane 0.001; Freely soluble in chloroform, practically insoluble in ether /Verapamil hydrochloride/, Practically insoluble in water, Sparingly soluble in hexane; soluble in benzene, ether; freely soluble in the lower alcohols, acetone, ethyl acetate, chloroform, 4.47 mg/L | |
| Record name | Verapamil | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3928 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Verapamil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001850 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Verapamil inhibits L-type calcium channels by binding to a specific area of their alpha-1 subunit,Cav1.2, which is highly expressed on L-type calcium channels in vascular smooth muscle and myocardial tissue where these channels are responsible for the control of peripheral vascular resistance and heart contractility. Calcium influx through these channels allows for the propagation of action potentials necessary for the contraction of muscle tissue and the heart's electrical pacemaker activity. Verapamil binds to these channels in a voltage- and frequency-dependent manner, meaning affinity is increased 1) as vascular smooth muscle membrane potential is reduced, and 2) with excessive depolarizing stimulus. Verapamil's mechanism of action in the treatment of angina and hypertension is likely due to the mechanism described above. Inhibition of calcium influx prevents the contraction of vascular smooth muscle, causing relaxation/dilation of blood vessels throughout the peripheral circulation - this lowers systemic vascular resistance (i.e. afterload) and thus blood pressure. This reduction in vascular resistance also reduces the force against which the heart must push, decreasing myocardial energy consumption and oxygen requirements and thus alleviating angina. Electrical activity through the AV node is responsible for determining heart rate, and this activity is dependent upon calcium influx through L-type calcium channels. By inhibiting these channels and decreasing the influx of calcium, verapamil prolongs the refractory period of the AV node and slows conduction, thereby slowing and controlling the heart rate in patients with arrhythmia. Verapamil's mechanism of action in the treatment of cluster headaches is unclear, but is thought to result from an effect on other calcium channels (e.g. N-, P-, Q-, or T-type). Verapamil is known to interact with other targets, including other calcium channels, potassium channels, and adrenergic receptors., Calcium antagonists inhibit excitation-contraction coupling in myocardial and smooth muscle by blocking the transmembrane carrier of calcium. This results in decreased myocardial contractility and in vasodilatation. ... /Salt not specified/, Verapamil has been shown to be neuroprotective in several acute neurotoxicity models due to blockade of calcium entry into neurons. However, the potential use of verapamil to treat chronic neurodegenerative diseases has not been reported. Using rat primary mesencephalic neuron/glia cultures, we report that verapamil significantly inhibited LPS-induced dopaminergic neurotoxicity in both pre- and post-treatment experiments. Reconstituted culture studies revealed that the presence of microglia was essential in verapamil-elicited neuroprotection. Mechanistic studies showed that decreased production of inflammatory mediators from LPS-stimulated microglia underlay neuroprotective property of verapamil. Further studies demonstrated that microglial NADPH oxidase (PHOX), the key superoxide-producing enzyme, but not calcium channel in neurons, is the site of action for the neuroprotective effect of verapamil. This conclusion was supported by the following two observations: 1) Verapamil failed to show protective effect on LPS-induced dopaminergic neurotoxicity in PHOX-deficient (deficient in the catalytic subunit of gp91(phox)) neuron/glia cultures; 2) Ligand binding studies showed that the binding of (3)H-verapamil onto gp91(phox) transfected COS7 cell membranes was higher than the non-transfected control. The calcium channel-independent neuroprotective property of verapamil was further supported by the finding that R(+)-verapamil, a less active form in blocking calcium channel, showed the same potency in neuroprotection, inhibition of pro-inflammatory factors production and binding capacity to gp91(phox) membranes as R(-)-verapamil, the active isomer of calcium channel blocker. In conclusion, our results demonstrate a new indication of verapamil-mediated neuroprotection through a calcium channel-independent pathway and provide a valuable avenue for the development of therapy for inflammation-related neurodegenerative diseases. | |
| Record name | Verapamil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00661 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Verapamil | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3928 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Viscous, pale yellow oil | |
CAS No. |
52-53-9 | |
| Record name | Verapamil | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=52-53-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Verapamil [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000052539 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Verapamil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00661 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | (+/-)-Verapamil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9041152 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Verapamil | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.133 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | VERAPAMIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/CJ0O37KU29 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Verapamil | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3928 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Verapamil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001850 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
< 25 °C | |
| Record name | Verapamil | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0001850 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is Verapamil's primary molecular target?
A1: Verapamil primarily targets L-type voltage-gated calcium channels (Cav1.2) [, , , ]. These channels play a crucial role in calcium influx, influencing various physiological processes, particularly in cardiac and smooth muscle cells.
Q2: How does Verapamil interact with L-type calcium channels?
A2: Verapamil exhibits a higher affinity for the inactivated state of L-type calcium channels []. Binding to these channels, Verapamil inhibits calcium influx, leading to a decrease in intracellular calcium concentration.
Q3: What are the downstream effects of Verapamil's interaction with its target?
A3: By inhibiting calcium influx through L-type channels, Verapamil exerts several downstream effects, including:
- Cardiac: Decreased heart rate, reduced myocardial contractility, and prolonged atrioventricular (AV) nodal conduction time [, , , ].
- Vascular: Relaxation of vascular smooth muscle, leading to vasodilation and reduced blood pressure [, , ].
Q4: What is the molecular formula and weight of Verapamil?
A4: Verapamil's molecular formula is C27H38N2O4, and its molecular weight is 454.6 g/mol.
Q5: What is the bioavailability of orally administered Verapamil?
A5: Oral Verapamil has a low and variable bioavailability, averaging around 22% due to significant first-pass metabolism [].
Q6: How does Verapamil's metabolism impact its pharmacokinetic profile?
A6: Verapamil undergoes extensive hepatic metabolism, primarily by cytochrome P450 enzymes, particularly CYP3A4 [, ]. This leads to the formation of several metabolites, including norVerapamil, which also possesses pharmacological activity [, ].
Q7: Does Verapamil interact with other drugs?
A7: Yes, Verapamil can interact with various drugs due to its effects on drug-metabolizing enzymes and P-glycoprotein. For example, it can increase digoxin concentrations [], potentially leading to toxicity.
Q8: What are the main clinical applications of Verapamil?
A8: Verapamil is clinically used for treating various cardiovascular conditions, including:
- Supraventricular tachycardia (SVT): Intravenous Verapamil effectively terminates AV nodal reentrant tachycardia [].
- Hypertension: Verapamil lowers blood pressure [, ] and was found to be as effective as hydrochlorothiazide in achieving target blood pressure but with fewer patients requiring combination therapy [].
- Angina pectoris: Verapamil improves exercise capacity and reduces angina symptoms [, , ].
- Hypertrophic cardiomyopathy: Verapamil improves hemodynamic parameters and exercise capacity in patients with this condition [, ].
Q9: Are there differences in Verapamil's efficacy in different patient populations?
A9: Research suggests potential differences in Verapamil's effectiveness among different patient groups:
- Age: In elderly patients with ischemic heart disease, Verapamil was less effective than Diltiazem in increasing exercise duration, although it improved ischemic thresholds [].
- Training Status: Studies on sweating responses showed that L-type calcium channel blockade with Verapamil had a more pronounced effect on cholinergic sweating in trained individuals compared to untrained individuals [].
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.













