Proguanil
Descripción general
Descripción
Proguanil D6: es un derivado marcado con deuterio de this compound, un agente antimalárico. El this compound se utiliza ampliamente para la profilaxis y el tratamiento de la malaria causada por Plasmodium falciparum y Plasmodium vivax . El marcaje con deuterio en this compound D6 mejora su estabilidad y permite estudios farmacocinéticos más precisos .
Mecanismo De Acción
El Proguanil D6 ejerce sus efectos inhibiendo la enzima dihidrofolato reductasa, que es esencial para la reproducción del parásito de la malaria . Esta inhibición evita que el parásito sintetice ADN y se replique, deteniendo así la infección . El marcaje con deuterio en this compound D6 permite un seguimiento más preciso de la distribución y el metabolismo del fármaco en el cuerpo .
Análisis Bioquímico
Biochemical Properties
Proguanil is a biguanide derivative that is converted to an active metabolite called cycloguanil . It exerts its antimalarial action by inhibiting the enzyme, dihydrofolate reductase, which is involved in the reproduction of the malaria parasite, Plasmodium falciparum and Plasmodium vivax . This inhibition blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication .
Cellular Effects
This compound works by stopping the malaria parasite, Plasmodium falciparum and Plasmodium vivax, from reproducing once it is in the red blood cells . It does this by inhibiting the enzyme, dihydrofolate reductase, which is involved in the reproduction of the parasite . This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver .
Molecular Mechanism
The molecular mechanism of this compound involves the inhibition of the enzyme dihydrofolate reductase of plasmodia . This inhibition blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication . This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver .
Temporal Effects in Laboratory Settings
This compound has been shown to have potent, but slow-acting, in vitro anti-plasmodial activity . The potent fast-acting activity of this compound is attributed to the dihydrofolate reductase inhibitor cycloguanil .
Dosage Effects in Animal Models
While specific dosage effects of this compound in animal models were not found in the search results, it is known that this compound is extensively absorbed in rats . In both species, toxicity was related to this compound exposure, the principal manifestations being salivation, emesis, and loss of body weight .
Metabolic Pathways
This compound is variably metabolized in the liver by cytochrome P450 isoenzymes to the active triazine metabolite, cycloguanil . This variable metabolism of this compound may have profound clinical importance in poor metabolizers such as the Asian and African populations at risk for malaria infection .
Transport and Distribution
This compound and its metabolite cycloguanil were found to be substrates of organic cation transporter 1 (OCT1), organic cation transporter 2 (OCT2), multidrug and toxin extrusion 1 (MATE1) and multidrug and toxin extrusion 2-K (MATE2-K) . These transporters play a crucial role in the distribution and excretion of this compound .
Subcellular Localization
The specific subcellular localization of this compound was not found in the search results. Given its mechanism of action, it can be inferred that this compound likely localizes to the site of the enzyme dihydrofolate reductase, which is involved in the reproduction of the malaria parasite .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: : La síntesis de Proguanil D6 implica la incorporación de átomos de deuterio en la molécula de this compound. Esto se puede lograr mediante diversos métodos, incluyendo el uso de reactivos o disolventes deuterados durante el proceso de síntesis . Las condiciones de reacción suelen implicar el uso de disolventes deuterados, como dimetilsulfóxido deuterado (DMSO) o etanol deuterado, y las reacciones se llevan a cabo a temperaturas y presiones controladas para asegurar la incorporación de átomos de deuterio .
Métodos de producción industrial: : La producción industrial de this compound D6 sigue rutas sintéticas similares pero a mayor escala. El proceso implica el uso de reactores automatizados y un control preciso de las condiciones de reacción para garantizar un alto rendimiento y pureza del producto final .
Análisis De Reacciones Químicas
Tipos de reacciones: : El Proguanil D6 experimenta diversas reacciones químicas, que incluyen:
Oxidación: El this compound D6 se puede oxidar para formar su metabolito activo, cicloguanil.
Sustitución: Las reacciones de sustitución pueden ocurrir en el grupo cloro, lo que lleva a la formación de diferentes derivados.
Reactivos y Condiciones Comunes
Sustitución: Las reacciones de sustitución a menudo implican nucleófilos como aminas o tioles en condiciones básicas.
Productos Principales
Cicloguanil: El producto principal formado por la oxidación de this compound D6.
Varios derivados: Formados mediante reacciones de sustitución.
Aplicaciones Científicas De Investigación
Clinical Efficacy
Proguanil is most commonly administered in combination with atovaquone, marketed as Malarone. This combination has demonstrated high efficacy rates in preventing and treating malaria. For instance, a randomized placebo-controlled study showed that this combination was 100% effective in preventing malaria among children living in endemic areas . Another study highlighted that this compound effectively sensitizes malaria parasites to atovaquone, thereby enhancing treatment outcomes even in cases where resistance to other antimalarials is present .
Table 1: Efficacy Data from Clinical Studies
Case Studies
- Atovaquone/Proguanil-Induced Esophageal Ulcers : A case report documented a healthy medical student who developed esophageal ulcers after taking atovaquone/proguanil without water. This incident underscores the importance of proper administration methods for medications .
- Treatment of Imported Malaria : In a study involving travelers returning from endemic regions, atovaquone/proguanil was successfully used to treat multiple cases of P. falciparum and P. vivax malaria. The treatment was effective even in patients who had previously failed other treatments .
- Resistance Cases : A cluster of malaria cases treated with atovaquone/proguanil revealed resistance mutations in the P. falciparum genome. This highlights the ongoing challenge of drug resistance and the need for continuous monitoring and development of new treatment strategies .
Future Applications
Research is ongoing to explore additional applications of this compound beyond malaria treatment. Its potential as an antifungal agent is being investigated, particularly for use in immunocompromised patients at risk for fungal infections . Furthermore, this compound's role in combination therapies with other antimalarials continues to be a focus area due to its ability to enhance the efficacy of existing drugs.
Comparación Con Compuestos Similares
Compuestos Similares
Proguanil: El compuesto original de this compound D6, utilizado para la profilaxis y el tratamiento de la malaria.
Cicloguanil: El metabolito activo de this compound, formado por oxidación.
Cloroquina: Otro fármaco antimalárico que se utiliza a menudo en combinación con this compound.
Singularidad: : El this compound D6 es único debido a su marcaje con deuterio, que mejora su estabilidad y permite estudios farmacocinéticos más precisos en comparación con sus homólogos no deuterados . Esto lo convierte en una herramienta valiosa en la investigación científica y el desarrollo de fármacos.
Actividad Biológica
Proguanil is an antimalarial compound primarily used in the prevention and treatment of malaria, particularly against Plasmodium falciparum and Plasmodium vivax . Its biological activity is closely linked to its metabolism into the active metabolite cycloguanil, which exerts significant effects on the malaria parasites. This article explores the biological activity of this compound, including its mechanisms of action, pharmacokinetics, clinical efficacy, and safety profile.
This compound functions as a dihydrofolate reductase (DHFR) inhibitor , which is crucial for the biosynthesis of purines and pyrimidines necessary for DNA synthesis in malaria parasites. The inhibition of DHFR leads to a failure in nuclear division during the schizont formation phase within erythrocytes and liver cells .
Key Mechanisms:
- Inhibition of Dihydrofolate Reductase : this compound and its metabolite cycloguanil inhibit DHFR in malaria parasites, disrupting folate metabolism essential for DNA replication .
- Synergistic Action with Atovaquone : When combined with atovaquone (as in Malarone), this compound enhances the efficacy against resistant strains of malaria by targeting different pathways in the parasite's lifecycle .
Pharmacokinetics
This compound is rapidly absorbed following oral administration, with peak plasma concentrations occurring within 1-3 hours. It has a high bioavailability (approximately 75%) and is extensively metabolized in the liver to cycloguanil via cytochrome P450 enzymes (CYP2C19) .
Pharmacokinetic Parameters:
| Parameter | Value |
|---|---|
| Absorption | Rapid and well absorbed |
| Bioavailability | ~75% |
| Protein Binding | ~75% |
| Metabolism | Hepatic (CYP2C19) |
| Elimination Half-life | 8-10 hours |
Clinical Efficacy
Numerous studies have demonstrated the high efficacy of this compound, particularly when used in combination with atovaquone. A systematic review indicated that this combination therapy has a prophylactic efficacy of approximately 95.8% against malaria .
Case Studies:
- Study on Children : In a randomized controlled trial involving 320 children in Gabon, none of the children receiving atovaquone-proguanil developed positive blood smears during chemosuppression, compared to 25 cases in the placebo group (p<0.001)【6】【8】.
- Efficacy Against Resistant Strains : this compound has shown effectiveness even in regions where resistance to other antimalarial drugs is prevalent. For example, high antimalarial efficacy was observed in patients with poor metabolizer genotypes for CYP2C19【4】【5】.
Safety Profile
This compound is generally well-tolerated, with a lower incidence of adverse effects compared to alternative treatments. Common side effects include gastrointestinal disturbances such as nausea and vomiting【5】【6】. A meta-analysis reported fewer treatment-related adverse events leading to discontinuation among patients taking atovaquone-proguanil compared to those on other regimens【2】.
Adverse Effects Overview:
| Adverse Effect | Incidence (%) |
|---|---|
| Nausea | 33% |
| Vomiting | 29% |
| Abdominal Pain | Varies |
Propiedades
Key on ui mechanism of action |
Proguanil inhibits the dihydrofolate reductase of plasmodia and thereby blocks the biosynthesis of purines and pyrimidines, which are essential for DNA synthesis and cell multiplication. This leads to failure of nuclear division at the time of schizont formation in erythrocytes and liver. |
|---|---|
Número CAS |
500-92-5 |
Fórmula molecular |
C11H16ClN5 |
Peso molecular |
259.77 g/mol |
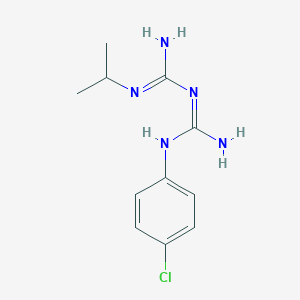
Nombre IUPAC |
1-[amino-(4-chloroanilino)methylidene]-2-(1,1,1,3,3,3-hexadeuteriopropan-2-yl)guanidine |
InChI |
InChI=1S/C11H16ClN5/c1-7(2)15-10(13)17-11(14)16-9-5-3-8(12)4-6-9/h3-7H,1-2H3,(H5,13,14,15,16,17)/i1D3,2D3 |
Clave InChI |
SSOLNOMRVKKSON-WFGJKAKNSA-N |
SMILES |
CC(C)N=C(N)N=C(N)NC1=CC=C(C=C1)Cl |
SMILES isomérico |
[2H]C([2H])([2H])C(C([2H])([2H])[2H])N=C(N)N=C(N)NC1=CC=C(C=C1)Cl |
SMILES canónico |
CC(C)N=C(N)N=C(N)NC1=CC=C(C=C1)Cl |
melting_point |
129 °C |
Key on ui other cas no. |
500-92-5 |
Descripción física |
Solid |
Pureza |
> 95% |
Cantidad |
Milligrams-Grams |
Números CAS relacionados |
637-32-1 (hydrochloride) |
Solubilidad |
2.86e-01 g/L |
Sinónimos |
Bigumal Chlorguanid Chloriguane Chloroguanide Chloroguanide Hydrochloride Hydrochloride, Chloroguanide Hydrochloride, Proguanil Paludrin Paludrine Proguanil Proguanil Hydrochloride |
Origen del producto |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does Proguanil exert its antimalarial effect?
A1: this compound itself has weak antimalarial activity. Its effectiveness stems from its active metabolite, Cycloguanil, a potent inhibitor of dihydrofolate reductase (DHFR) []. DHFR is a crucial enzyme in the folate metabolic pathway, essential for DNA synthesis and cellular replication in parasites like Plasmodium falciparum []. By inhibiting DHFR, Cycloguanil disrupts DNA synthesis and ultimately kills the parasite [, ].
Q2: Are there other mechanisms by which this compound impacts Plasmodium falciparum?
A2: Research suggests this compound, in combination with Atovaquone, might interfere with mitochondrial electron transport and collapse mitochondrial membrane potential in the parasite, further contributing to its antimalarial activity [].
Q3: Does this compound affect other stages of the Plasmodium life cycle besides the erythrocytic stage?
A3: Yes, both this compound and Atovaquone demonstrate activity against gametocytes and pre-erythrocytic (hepatic) stages of malaria parasites []. This is supported by studies indicating that short-term this compound administration might provide causal prophylaxis for Plasmodium vivax by inhibiting liver-stage schizonts, although it doesn't seem to prevent late attacks related to hypnozoite reactivation [].
Q4: How is this compound metabolized in the human body?
A4: this compound is primarily metabolized in the liver by cytochrome P450 (CYP) enzymes, specifically CYP2C19 and CYP3A4 [, ]. The primary metabolic pathway involves CYP2C19-mediated conversion to its active metabolite, Cycloguanil [, ].
Q5: What factors contribute to the variability in this compound metabolism among individuals?
A5: Inter-individual variability in this compound metabolism is influenced by several factors, primarily genetic polymorphisms in the CYP2C19 gene [, ]. Individuals homozygous for the CYP2C19*2 allele exhibit significantly reduced metabolic capacity, leading to higher this compound and lower Cycloguanil levels []. Other factors include co-administration of drugs that are CYP2C19 inducers or inhibitors [], and variations in the expression and activity of other enzymes involved in this compound metabolism, like CYP3A4 [].
Q6: How is this compound eliminated from the body?
A6: Both this compound and Cycloguanil are predominantly eliminated through the kidneys []. Therefore, dosage adjustments are necessary for patients with renal impairment to prevent drug accumulation [].
Q7: Are there documented cases of resistance to this compound?
A7: Yes, this compound resistance has been observed and is primarily attributed to point mutations in the dihydrofolate reductase (DHFR) gene of Plasmodium falciparum [, ]. The S108N mutation is particularly associated with this compound resistance [, ].
Q8: Is there cross-resistance between this compound and other antimalarial drugs?
A8: Yes, cross-resistance has been observed between this compound and Pyrimethamine, another antifolate drug []. This is attributed to their shared mechanism of action, both targeting the DHFR enzyme in the parasite. The presence of the triple mutant DHFR haplotype (S108N+N51I+C59N) in Plasmodium falciparum has been linked to resistance to both drugs [, ].
Q9: Does this compound interact with other drugs?
A9: Yes, this compound's metabolism can be affected by co-administration with other drugs metabolized by CYP2C19, such as Phenytoin []. Concomitant use of Phenytoin, a CYP2C19 inducer, can decrease this compound's area under the curve (AUC) and maximum concentration (Cmax), potentially impacting its efficacy [].
Q10: Beyond malaria, are there other potential therapeutic applications for this compound?
A10: Emerging research suggests that this compound may have anti-cancer properties, particularly in breast cancer. Studies have shown that this compound inhibits the growth of breast cancer cells in vitro and in vivo, potentially by inducing oxidative stress, disrupting mitochondrial function, and triggering apoptosis [, ].
Q11: What are the key considerations in formulating this compound for therapeutic use?
A11: this compound formulations aim to optimize solubility, bioavailability, and stability []. The choice of excipients and manufacturing processes can significantly influence these factors. For instance, some herbal formulations may significantly impact the dissolution profile of this compound tablets, potentially altering its bioavailability and warranting further investigation for potential herb-drug interactions [].
Q12: What analytical techniques are commonly employed to quantify this compound and its metabolites?
A12: High-performance liquid chromatography (HPLC) is widely used to measure this compound and its metabolites in biological samples like plasma and urine [, , ]. Ultra-performance liquid chromatography (UPLC) offers enhanced speed and sensitivity for pharmacokinetic studies [].
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.















