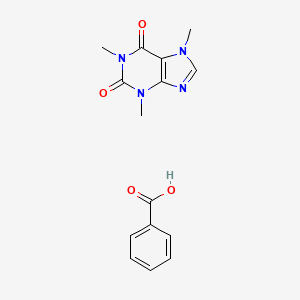
Caffeine benzoate
- Cliquez sur DEMANDE RAPIDE pour recevoir un devis de notre équipe d'experts.
- Avec des produits de qualité à un prix COMPÉTITIF, vous pouvez vous concentrer davantage sur votre recherche.
Vue d'ensemble
Description
Caffeine benzoate, also known as this compound, is a useful research compound. Its molecular formula is C15H16N4O4 and its molecular weight is 316.31 g/mol. The purity is usually 95%.
The exact mass of the compound this compound is unknown and the complexity rating of the compound is unknown. The storage condition is unknown. Please store according to label instructions upon receipt of goods.Use and application categories indicated by third-party sources: Cosmetics -> Skin conditioning. However, this does not mean our product can be used or applied in the same or a similar way.
BenchChem offers high-quality this compound suitable for many research applications. Different packaging options are available to accommodate customers' requirements. Please inquire for more information about this compound including the price, delivery time, and more detailed information at [email protected].
Applications De Recherche Scientifique
Dermatological Applications
Caffeine Benzoate as a Sunscreen Agent
This compound has been studied for its potential as a topical agent that enhances UV protection. Research indicates that it significantly inhibits UVB-induced skin damage, including the formation of thymine dimers, which are precursors to skin cancer. In studies involving SKH-1 hairless mice, topical application of this compound resulted in:
- 90% inhibition of UVB-induced thymine dimers.
- Enhanced apoptosis in UVB-irradiated skin, with this compound showing a 2- to 3-fold greater effect compared to caffeine alone .
Tumor Prevention
This compound has demonstrated efficacy in preventing skin tumors induced by UV radiation. In chronic UVB exposure studies, it was found to inhibit tumor formation and reduce tumor size significantly:
- 76% reduction in the total number of skin tumors per mouse with a 6% concentration of this compound.
- 88% decrease in the number of squamous cell carcinomas .
Psychiatric Applications
Augmentation in Electroconvulsive Therapy
Recent studies have explored the use of this compound as an augmentation agent in electroconvulsive therapy (ECT). In a clinical trial involving 71 patients:
- Administration of 500 mg this compound increased seizure duration by an average of 24.1 seconds , enhancing the efficacy of ECT.
- Patients receiving this compound exhibited improved seizure response rates, which is crucial for effective treatment outcomes .
Neurobehavioral Effects
Impact on Adolescent Behavior
Research has also investigated the neurobehavioral effects of this compound when combined with sodium benzoate in adolescent rats. Key findings include:
- Increased motor activity and improved recognition memory associated with sodium benzoate and caffeine co-administration.
- However, adverse effects on working memory were observed at higher doses, indicating potential risks when combined with processed foods containing sodium benzoate .
Data Summary Table
| Application Area | Findings | Concentration/Method |
|---|---|---|
| Dermatology | 90% inhibition of thymine dimers; enhanced apoptosis | Topical application (6% solution) |
| 76% reduction in skin tumors | Chronic UVB exposure studies | |
| Psychiatry | Increased seizure duration by 24.1 seconds during ECT | Intravenous administration (500 mg) |
| Neurobehavioral Effects | Improved motor activity; recognition memory enhancement | Adolescent rat studies |
Case Studies
-
Skin Cancer Prevention Study
- Objective: To evaluate the effectiveness of this compound in preventing UVB-induced skin tumors.
- Results: Significant reductions in both tumor incidence and size were observed with regular topical applications over an extended period.
-
ECT Augmentation Study
- Objective: To assess the impact of this compound on seizure duration during ECT.
- Results: Patients demonstrated increased seizure durations and improved clinical outcomes compared to those not receiving this compound.
Propriétés
Numéro CAS |
5743-17-9 |
|---|---|
Formule moléculaire |
C15H16N4O4 |
Poids moléculaire |
316.31 g/mol |
Nom IUPAC |
benzoic acid;1,3,7-trimethylpurine-2,6-dione |
InChI |
InChI=1S/C8H10N4O2.C7H6O2/c1-10-4-9-6-5(10)7(13)12(3)8(14)11(6)2;8-7(9)6-4-2-1-3-5-6/h4H,1-3H3;1-5H,(H,8,9) |
Clé InChI |
VXXKVQICUHMECR-UHFFFAOYSA-N |
SMILES |
CN1C=NC2=C1C(=O)N(C(=O)N2C)C.C1=CC=C(C=C1)C(=O)O |
SMILES canonique |
CN1C=NC2=C1C(=O)N(C(=O)N2C)C.C1=CC=C(C=C1)C(=O)O |
Key on ui other cas no. |
5743-17-9 |
Numéros CAS associés |
8000-95-1 (hydrochloride salt) |
Synonymes |
caffeine - sodium benzoate caffeine benzoate caffeine, sodium benzoate drug combination |
Origine du produit |
United States |
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.
















